16.5 Neutralization
Learning Objectives
- Describe a neutralization reaction.
- Predict whether a salt solution will be acidic, basic, or neutral.
- Perform calculations involving titrations of strong acids and strong bases.
As we have seen in the section on chemical reactions, when an acid and base are mixed, they undergo a neutralization reaction. The word “neutralization” seems to imply that a stoichiometrically equivalent solution of an acid and a base would be neutral. This is sometimes true, but the salts that are formed in these reactions may have acidic or basic properties of their own that affect the overall pH.
Acid-Base Neutralization
Recall the general chemical equation for a neutralization reaction:
Acid + Base → Salt + Water
It is important to remember neutralization reactions are just a specific type of double displacement reaction.
A solution is neutral when it contains equal concentrations of hydronium and hydroxide ions. When we mix solutions of an acid and a base, an acid-base neutralization reaction occurs. However, even if we mix stoichiometrically equivalent quantities, we may find that the resulting solution is not neutral. It could contain either an excess of hydronium ions or an excess of hydroxide ions because the nature of the salt formed determines whether the solution is acidic, neutral, or basic. The following four situations illustrate how solutions with various pH values can arise following a neutralization reaction using stoichiometrically equivalent quantities:
- A strong acid and a strong base, such as HCl(aq) and NaOH(aq), react to form a neutral solution since they fully dissociate in water and their conjugate partners produced are of negligible strength:
[latex]\text{HCl}(aq)\;+\;\text{NaOH}(aq)\;{\rightleftharpoons}\;\text{NaCl}(aq)\;+\;\text{H}_2\text{O}(l)[/latex]
- A strong acid and a weak base yield a weakly acidic solution, not because of the strong acid involved, but because of the conjugate acid of the weak base. A weak base produces a strong conjugate acid, and this will affect the overall pH to be more acidic.
- A weak acid and a strong base yield a weakly basic solution. A solution of a weak acid reacts with a solution of a strong base to form the conjugate base of the weak acid and the conjugate acid of the strong base. The conjugate acid of the strong base is a weaker acid than water and has no effect on the acidity of the resulting solution. However, the conjugate base of the weak acid is a weak base and ionizes slightly in water. This increases the amount of hydroxide ion in the solution produced in the reaction and renders it slightly basic.
- A weak acid plus a weak base can yield either an acidic, basic, or neutral solution. This is the most complex of the four types of reactions. When the conjugate acid and the conjugate base are of unequal strengths, the solution can be either acidic or basic, depending on the relative strengths of the two conjugates. Occasionally the weak acid and the weak base will have the same strength, so their respective conjugate base and acid will have the same strength, and the solution will be neutral. To predict whether a particular combination will be acidic, basic or neutral, tabulated K values of the conjugates must be compared.
- The acid dissociation constant, (Ka), is an equilibrium constant that gives numerical representation of an acid’s strength in a solution based its degree of dissociation in water. The greater the Ka, the stronger the acid.
- The base dissociation constant, (Kb), is an equilibrium constant that measures how completely a base dissociates into ions in water. The greater the Kb, the stronger the base.
Stomach Antacids
Our stomachs contain a solution of roughly 0.03 M HCl, which helps us digest the food we eat. The burning sensation associated with heartburn is a result of the acid of the stomach leaking through the muscular valve at the top of the stomach into the lower reaches of the esophagus. The lining of the esophagus is not protected from the corrosive effects of stomach acid the way the lining of the stomach is, and the results can be very painful. When we have heartburn, it feels better if we reduce the excess acid in the esophagus by taking an antacid. As you may have guessed, antacids are bases. One of the most common antacids is calcium carbonate, CaCO3. The reaction,
not only neutralizes stomach acid, it also produces CO2(g), which may result in a satisfying belch.
Milk of Magnesia is a suspension of the sparingly soluble base magnesium hydroxide, Mg(OH)2. It works according to the reaction:
The hydroxide ions generated in this equilibrium then go on to react with the hydronium ions from the stomach acid, so that :
This reaction does not produce carbon dioxide, but magnesium-containing antacids can have a laxative effect.
Several antacids have aluminum hydroxide, Al(OH)3, as an active ingredient. The aluminum hydroxide tends to cause constipation, and some antacids use aluminum hydroxide in coordination with magnesium hydroxide to balance the side effects of the two substances.

Culinary Aspects of Chemistry
Cooking is essentially synthetic chemistry that happens to be safe to eat. There are a number of examples of acid-base chemistry in the culinary world. One example is the use of baking soda, or sodium bicarbonate in baking. NaHCO3 is a base. When it reacts with an acid such as lemon juice, buttermilk, or sour cream in a batter, bubbles of carbon dioxide gas are formed from decomposition of the resulting carbonic acid, and the batter “rises.” Baking powder is a combination of sodium bicarbonate, and one or more acid salts that react when the two chemicals come in contact with water in the batter.
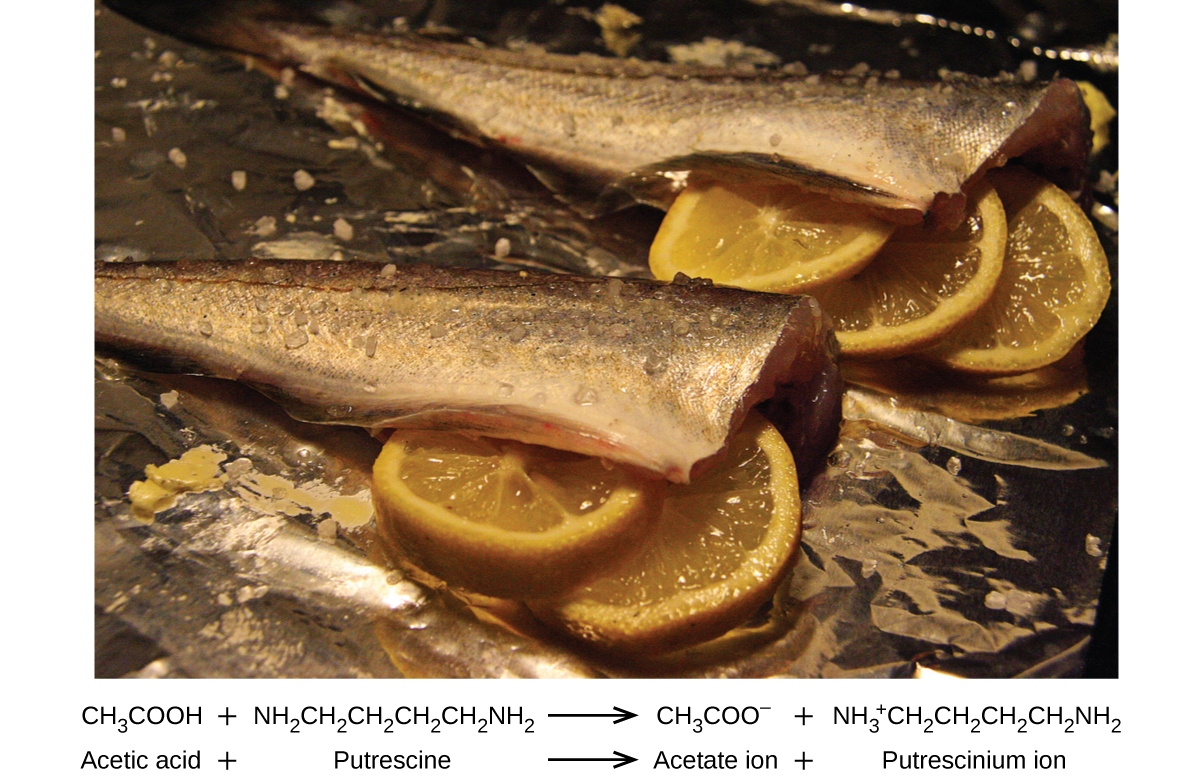
Many people like to put lemon juice or vinegar, both of which are acids, on cooked fish (Figure 16.5b). It turns out that fish have volatile amines (bases) in their systems, which are neutralized by the acids to yield involatile ammonium salts. This reduces the odour of the fish, and also adds a “sour” taste that we seem to enjoy.
Pickling is a method used to preserve vegetables using a naturally produced acidic environment. The vegetable, such as a cucumber, is placed in a sealed jar submerged in a brine solution. The brine solution favours the growth of beneficial bacteria and suppresses the growth of harmful bacteria. The beneficial bacteria feed on starches in the cucumber and produce lactic acid as a waste product in a process called fermentation. The lactic acid eventually increases the acidity of the brine to a level that kills any harmful bacteria, which require a basic environment. Without the harmful bacteria consuming the cucumbers they are able to last much longer than if they were unprotected. A byproduct of the pickling process changes the flavour of the vegetables with the acid making them taste sour.
These cooking practices emerged from Peoples of the past. For example, many of these modern-day preparation and cooking practices steam from traditional Indigenous ingredients and techniques.
Indigenous Perspective: Indigenous Food Culture

Launched in 2020 by the Indigenous Tourism Association of Canada (ITAC), the website, Destination Indigenous, brings awareness and mindfulness to the best Indigenous tourism experiences in Canada from Pacific coast to Arctic coast to Atlantic coast, including Indigenous food culture.
In an excerpt from Food & Culture - Indigenous Cuisine website (2020), food as part of an interdependent ecosystem is highlighted:
"Traditional food sources were seen as part of a healthy and interdependent ecosystem. Indigenous [communities] traditionally only harvested, hunted or gathered what they needed to survive, and endeavoured to not let anything go to waste. In communities with abundant fish, for example, every edible part of the fish was eaten, including the head, eyes, offal and eggs.
Inedible animal or plant material was often ingeniously repurposed for practical use. Animal bones could be used for tools, tanned hides and furs could be used for shelter and clothing, rawhide could be used for snowshoes, fishing nets or drum covers, and intestines or bladders could be used for cooking vessels or water storage. Plant materials, like spruce root or birch bark, could also be used for food storage." (Food & Culture - Indigenous Cuisine, 2020, para. 4).
Strong Acid-Strong Base Reactions
When a strong acid and a strong base are combined in stoichiometrically equivalent quantities - when [H+] equals [OH−] - a neutral solution results (pH = 7). The acid and base have neutralized each other, and the acidic and basic properties are no longer present. When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the result is a neutral solution. The products of the reaction do not have the characteristics of either an acid or a base. The balanced molecular equation is:
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
As discussed in chapter 14, chemical reactions occurring in aqueous solution are more accurately represented with a net ionic equation. The full ionic equation for the neutralization of hydrochloric acid by sodium hydroxide is written as follows:
H+(aq) + Cl−(aq) + Na+(aq) + OH−(aq) → Na+(aq) + Cl−(aq) + H2O(l)
Since the acid and base are both strong, they are fully ionized and so are written as ions, as is the NaCl formed as a product. The sodium and chloride ions are spectator ions in the reaction, leaving the following as the net ionic reaction.
H+ (aq) + OH−(aq) → H2O(l)
All neutralization reactions of a strong acid with a strong base simplify to the net ionic reaction of hydrogen ion combining with hydroxide ion to produce water.
Watch Acid-Base Reaction (HCl + NaOH) (0min 56s).
Video Source: BerkeleyChemDemos (2013, Oct 1). Acid-base reaction (HCl + NaOH). [Video]. YouTube.
What if the acid is a diprotic acid such as sulfuric acid? The balanced molecular equation now involves a 1:2 ratio between acid and base.
H2SO4(aq) + 2NaOH(aq) → Na2SO4(aq) + H2O(l)
In order for the reaction to be a full neutralization, twice as many moles of NaOH must react with the H2SO4. The sodium sulfate salt is soluble, and so the net ionic reaction is again the same. Different mole ratios occur for other polyprotic acids or bases with multiple hydroxides such as Ca(OH)2.
Reactions Involving a Weak Acid or Weak Base
Salt solutions do not always neutralize to have a pH of 7. As a general concept, if a strong acid is mixed with a weak base, the resulting solution will be slightly acidic. If a strong base is mixed with a weak acid, the solution will be slightly basic. The reaction between weak nitrous acid and strong potassium hydroxide is shown below.
HNO2(aq) + KOH(aq) → KNO2(aq) + H2O(l)
In order to write the net ionic equation, the weak acid must be written as a molecule since it does not ionize to a great extent in water. The base and the salt are fully dissociated.
HNO2(aq) + K+(aq) + OH−(aq) → K+(aq) + NO2-(aq) + H2O(l)
The only spectator ion is the potassium ion, resulting in the net ionic equation:
HNO2(aq) + OH−(aq) → NO2-(aq) + H2O(l)
The strong hydroxide ion essentially "forces" the weak nitrous acid to become ionized. The hydrogen ion from the acid combines with the hydroxide ion to form water, leaving the nitrite ion as the other product. The resulting solution is not neutral, but instead is slightly basic.
Reactions can also involve a weak base and strong acid, resulting in a solution that is slightly acidic. The molecular and net ionic equations for the reaction of hydrochloric acid and ammonia are shown below.
HCl(aq) + NH3(aq) → NH4Cl(aq)
H+(aq) + NH3(aq) → NH4+(aq) (Cl−is a spectator ion)
Reactions between acids and bases that are both weak may result in solutions that are neutral, acidic, or basic.
Source: "Strong Acid-Strong Base Reactions" was adapted by Jackie MacDonald from "21.16: Neutralization Reaction and Net Ionic Equations for Neutralization Reactions" In Introductory Chemistry (CK-12) by CK-12 Foundation, shared under CK-12 license
Attributions & References
References
Food & Culture - Indigenous Cuisine. (2020, September 21). DI - Culinary.
is an equilibrium constant that gives numerical representation of an acid’s strength in a solution based its degree of dissociation in water. The greater the Ka, the stronger the acid.
is an equilibrium constant that measures how completely a base dissociates into ions in water. The greater the Kb, the stronger the base.
A diprotic acid is an acid that yields two H+ ions per acid molecule. Examples of diprotic acids are sulfuric acid, H2SO4, and carbonic acid, H2CO3

