7.2 Molecular Mass, Avogadro’s Number and The Mole
Learning Objectives
By the end of this section, you will be able to:
- Calculate molecular masses (or numerically equivalent molar masses) for covalent and ionic compounds
- Explain the relation between mass, moles, and numbers of atoms or molecules, and perform calculations deriving quantities from one another
We can argue that modern chemical science began when scientists started exploring the quantitative as well as the qualitative aspects of chemistry. For example, Dalton’s atomic theory was an attempt to explain the results of measurements that allowed him to calculate the relative masses of elements combined in various compounds. Understanding the relationship between the masses of atoms and the chemical formulas of compounds allows us to quantitatively describe the composition of substances.
Molecular Mass for Substances
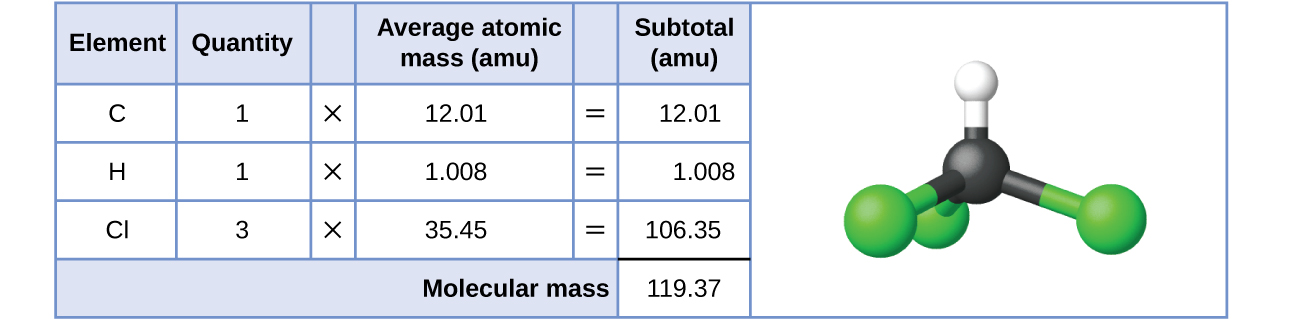
The formula represents the numbers and types of atoms composing a single molecule of the substance; therefore, the formula mass may be correctly referred to as a molecular mass. This value of the formula mass can also be used to consider a substance's molar mass (numerically equivalent to molecular mass per 1 mole of substance as discussed in section 7.1). Consider chloroform (CHCl3), a covalent compound once used as a surgical anesthetic and now primarily used in the production of the “anti-stick” polymer, Teflon. The molecular formula of chloroform indicates that a single molecule contains one carbon atom, one hydrogen atom, and three chlorine atoms. The average molar mass of a chloroform molecule is therefore equal to the sum of the average atomic masses of these atoms. Figure 7.2a outlines the calculations used to derive the molecular mass of chloroform, which is 119.37 amu. The molecular mass of 119.37 amu expressed as a molar mass is 119.37 g/mol.
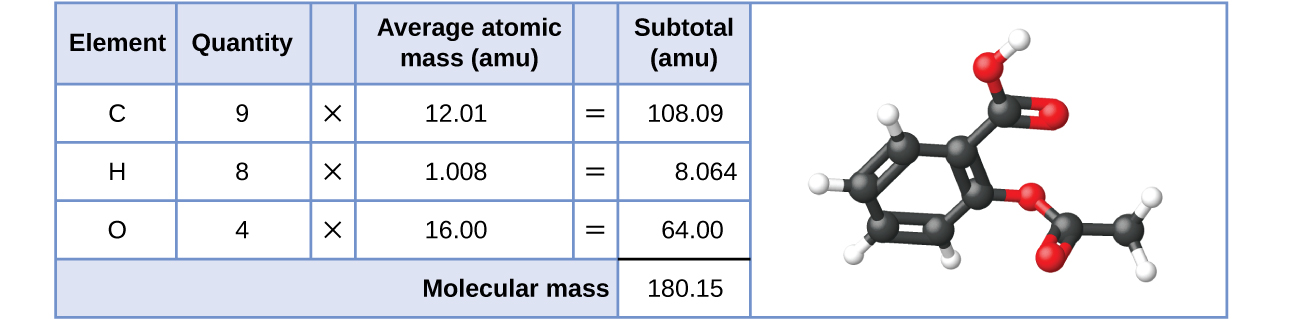
Likewise, the molecular mass of an aspirin molecule, C9H8O4, is the sum of the atomic masses of nine carbon atoms, eight hydrogen atoms, and four oxygen atoms, which amounts to 180.15 amu (Figure 7.2b) or 180.15 g/mol (molar mass).
Example 7.2a
Computing Molecular Mass for a Covalent Compound
Ibuprofen, C13H18O2, is a covalent compound and the active ingredient in several popular nonprescription pain medications, such as Advil and Motrin. What is the molecular mass (amu) for this compound? What is the molar mass?
Solution
Molecules of this compound are comprised of 13 carbon atoms, 18 hydrogen atoms, and 2 oxygen atoms. Following the approach described above, the average molecular mass for this compound is, therefore:
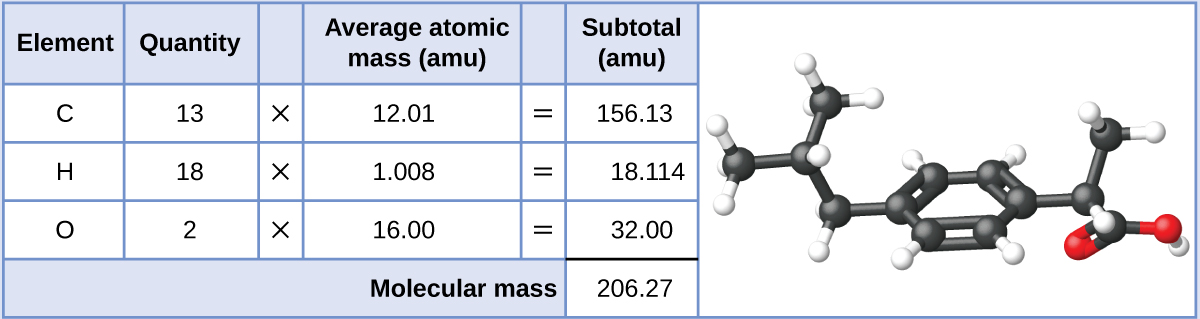
The molecular mass is 206.27 amu. The molar mass is 206.27 g/mol.
Exercise 7.2a
Acetaminophen, C8H9NO2, is a covalent compound and the active ingredient in several popular nonprescription pain medications, such as Tylenol. What is the molecular mass (amu) for this compound? What is the molar mass?
Check Your Answer[1]
Example 7.2b
Computing Molecular Mass for an Ionic Compound
Aluminum sulfate, Al2(SO4)3, is an ionic compound that is used in the manufacture of paper and in various water purification processes. What is the molecular mass (amu) of this compound? What is the molar mass?
Solution
The formula for this compound indicates it contains Al3+ and SO42− ions combined in a 2:3 ratio. For purposes of computing a formula mass, it is helpful to rewrite the formula in the simpler format, Al2S3O12. Following the approach outlined above, the formula mass for this compound is calculated as follows:
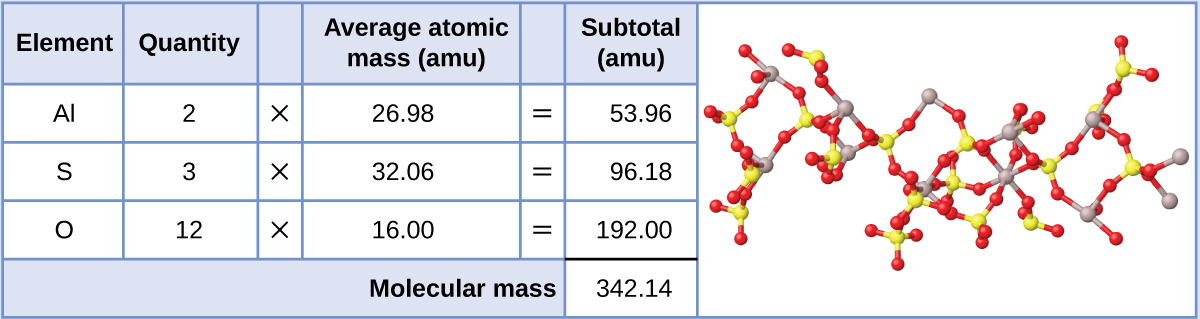
The molecular mass is 342.14 amu. The molar mass is 342.14 g/mol.
Exercise 7.2b
Calcium phosphate, Ca3(PO4)2, is an ionic compound and a common anti-caking agent added to food products. What is the molecular mass (amu) of calcium phosphate? What is the molar mass?
Check Your Answer[2]
The Mole
Recall that Avogadro’s number (NA) is a constant and is properly reported with an explicit unit of “per mole." Therefore, we can use Avogadro's number for counting molecules as well:
6.022 x 1023 molecules = 1 mole of molecules
Consistent with its definition as an amount unit, 1 mole of any compound contains the same number of molecules as 1 mole of any other compound. However, the molar mass of two different compounds will be different because their formula masses are different. The molar mass of a compound in grams is likewise numerically equivalent to its formula mass in amu (Figure 7.2c).

| Element | Average Atomic Mass (amu) | Molar Mass (g/mol) | Atoms/Mole |
|---|---|---|---|
| C | 12.01 | 12.01 | 6.022 × 1023 |
| H | 1.008 | 1.008 | 6.022 × 1023 |
| O | 16.00 | 16.00 | 6.022 × 1023 |
| Na | 22.99 | 22.99 | 6.022 × 1023 |
| Cl | 35.45 | 33.45 | 6.022 × 1023 |
While the formula mass and molar mass of a compound are numerically equivalent, keep in mind that they are vastly different in terms of scale, as represented by the vast difference in the magnitudes of their respective units (amu versus g). To appreciate the enormity of the mole, consider a small drop of water weighing about 0.03 g (see Figure 7.2d). Although this represents just a tiny fraction of 1 mole of water (~18 g), it contains more water molecules than can be clearly imagined. If the molecules were distributed equally among the roughly seven billion people on earth, each person would receive more than 100 billion molecules.

The relationships between formula mass, the mole, and Avogadro’s number can be applied to compute various quantities that describe the composition of compounds. For example, if we know the mass and chemical composition of a substance, we can determine the number of moles and calculate a number of atoms or molecules in the sample. Likewise, if we know the number of moles of a substance, we can derive the number of atoms or molecules and calculate the substance’s mass.
These relationships can be represented mathematically using the following expressions:
[latex]n = \frac{m}{M}[/latex]
Example 7.2c
Deriving Moles from Grams for an Element
According to nutritional guidelines from the US Department of Agriculture, the estimated average requirement for dietary potassium is 4.7 g. What is the estimated average requirement of potassium in moles?
Solution
The mass of K is provided, and the corresponding amount of K in moles is requested. Referring to the periodic table, the atomic mass of K is 39.10 amu, and so its molar mass is 39.10 g/mol. The given mass of K (4.7 g) is a bit more than one-tenth the molar mass (39.10 g), so a reasonable “ballpark” estimate of the number of moles would be slightly greater than 0.1 mol.
The molar amount of a substance may be calculated by dividing its mass (g) by its molar mass (g/mol):

The factor-label method supports this mathematical approach since the unit “g” cancels and the answer has units of “mol:”
[latex]4.7 \;\rule[0.5ex]{1.5em}{0.1ex}\hspace{-1.5em}\text{g K} \times \frac{1 \;\text{mol K}}{39.10 \;\rule[0.5ex]{1.5em}{0.1ex}\hspace{-1.5em}\text{g K}} = 0.12 \;\text{mol K}[/latex]
The calculated magnitude (0.12 mol K) is consistent with our ballpark expectation, since it is a bit greater than 0.1 mol.
Exercise 7.2c
Beryllium is a light metal used to fabricate transparent X-ray windows for medical imaging instruments. How many moles of Be are in a thin-foil window weighing 3.24 g?
Check Your Answer[3]
Example 7.2d
Deriving Grams from Moles for an Element
A litre of air contains 9.2 × 10−4 mol argon. What is the mass of Ar in a litre of air?
Solution
The molar amount of Ar is provided and must be used to derive the corresponding mass in grams. Since the amount of Ar is less than 1 mole, the mass will be less than the mass of 1 mole of Ar, approximately 40 g. The molar amount in question is approximately one-one thousandth (~10−3) of a mole, and so the corresponding mass should be roughly one-one thousandth of the molar mass (~0.04 g):

In this case, logic dictates (and the factor-label method supports) multiplying the provided amount (mol) by the molar mass (g/mol):
[latex]9.2 \times 10^{-4} \;\rule[0.5ex]{3em}{0.1ex}\hspace{-3em}\text{mol Ar} \times \frac{39.95 \;\text{g Ar}}{1 \;\rule[0.5ex]{3em}{0.1ex}\hspace{-3em}\text{mol Ar}} = 0.037 \;\text{g Ar}[/latex]
The result is in agreement with our expectations, around 0.04 g Ar.
Exercise 7.2d
What is the mass of 2.561 mol of gold?
Check Your Answer[4]
Example 7.2e
Deriving Moles from Grams for a Compound
Our bodies synthesize protein from amino acids. One of these amino acids is glycine, which has the molecular formula C2H5O2N. How many moles of glycine molecules are contained in 28.35 g of glycine?
Solution
We can derive the number of moles of a compound from its mass by the following:
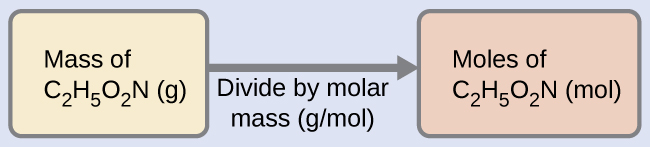
The molar mass of glycine is required for this calculation, and it is computed in the same fashion as its molecular mass. One mole of glycine, C2H5O2N, contains 2 moles of carbon, 5 moles of hydrogen, 2 moles of oxygen, and 1 mole of nitrogen:
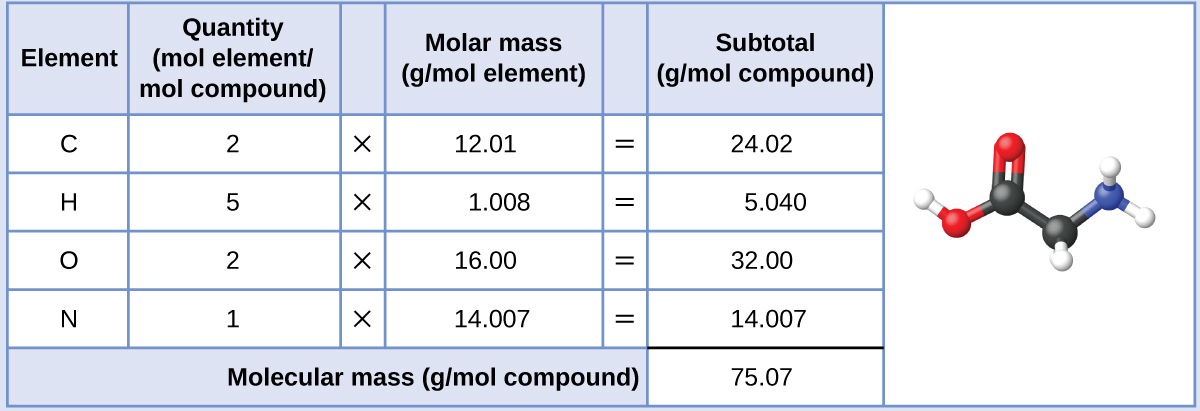
The provided mass of glycine (~28 g) is a bit more than one-third the molar mass (~75 g/mol), so we would expect the computed result to be a bit greater than one-third of a mole (~0.33 mol). Dividing the compound’s mass by its molar mass yields:
This result is consistent with our rough estimate.
Exercise 7.2e
How many moles of sucrose, C12H22O11, are in a 25-g sample of sucrose?
Check Your Answer[5]
Example 7.2f
Deriving Grams from Moles for a Compound
Vitamin C is a covalent compound with the molecular formula C6H8O6. The recommended daily dietary allowance of vitamin C for children aged 4–8 years is 1.42 × 10−4 mol. What is the mass of this allowance in grams?
Solution
As for elements, the mass of a compound can be derived from its molar amount as shown:

The molar mass for this compound is computed to be 176.124 g/mol. The given number of moles is a very small fraction of a mole (~10−4 or one-ten thousandth); therefore, we would expect the corresponding mass to be about one-ten thousandth of the molar mass (~0.02 g). Performing the calculation, we get:
This is consistent with the anticipated result.
Exercise 7.2f
What is the mass of 0.443 mol of hydrazine, N2H4?
Check Your Answer[6]
Example 7.2g
Deriving the Number of Atoms from Mass for an Element
Copper is commonly used to fabricate electrical wire (Figure 7.2e). How many copper atoms are in 5.00 g of copper wire?

Solution
The number of Cu atoms in the wire may be conveniently derived from its mass by a two-step computation: first calculating the molar amount of Cu, and then using Avogadro’s number (NA) to convert this molar amount to number of Cu atoms: Considering that the provided sample mass (5.00 g) is a little less than one-tenth the mass of 1 mole of Cu (~64 g), a reasonable estimate for the number of atoms in the sample would be on the order of one-tenth NA, or approximately 1022 Cu atoms. Carrying out the two-step computation yields:
Considering that the provided sample mass (5.00 g) is a little less than one-tenth the mass of 1 mole of Cu (~64 g), a reasonable estimate for the number of atoms in the sample would be on the order of one-tenth NA, or approximately 1022 Cu atoms. Carrying out the two-step computation yields:
[latex]5.00 \;\rule[0.5ex]{2em}{0.1ex}\hspace{-2em}\text{g Cu} \times \frac{1 \;\rule[0.5ex]{3em}{0.1ex}\hspace{-3em}\text{mol Cu}}{63.55 \;\rule[0.5ex]{2em}{0.1ex}\hspace{-2em}\text{g Cu}} \times \frac{6.022 \times 10^{23} \;\text{atoms Cu}}{1 \;\rule[0.5ex]{3em}{0.1ex}\hspace{-3em}\text{mol Cu}} = 4.74 \times 10^{22} \;\text{atoms Cu}[/latex]
The factor-label method yields the desired cancellation of units, and the computed result is on the order of 1022 as expected.
Exercise 7.2g
A prospector panning for gold in a river collects 15.00 g of pure gold. How many Au atoms are in this quantity of gold?
Check Your Answer[7]
Counting Neurotransmitter Molecules in the Brain
The brain is the control centre of the central nervous system (Figure 7.2f). It sends and receives signals to and from muscles and other internal organs to monitor and control their functions; it processes stimuli detected by sensory organs to guide interactions with the external world; and it houses the complex physiological processes that give rise to our intellect and emotions. The broad field of neuroscience spans all aspects of the structure and function of the central nervous system, including research on the anatomy and physiology of the brain. Great progress has been made in brain research over the past few decades, and the BRAIN Initiative, a federal initiative announced in 2013, aims to accelerate and capitalize on these advances through the concerted efforts of various industrial, academic, and government agencies.
Specialized cells called neurons transmit information between different parts of the central nervous system by way of electrical and chemical signals. Chemical signalling occurs at the interface between different neurons when one of the cells releases molecules (called neurotransmitters) that diffuse across the small gap between the cells (called the synapse) and bind to the surface of the other cell. These neurotransmitter molecules are stored in small intracellular structures called vesicles that fuse to the cell wall and then break open to release their contents when the neuron is appropriately stimulated. This process is called exocytosis (see Figure 7.2g). One neurotransmitter that has been very extensively studied is dopamine, C8H11NO2. Dopamine is involved in various neurological processes that impact a wide variety of human behaviours. Dysfunctions in the dopamine systems of the brain underlie serious neurological diseases such as Parkinson’s and schizophrenia.
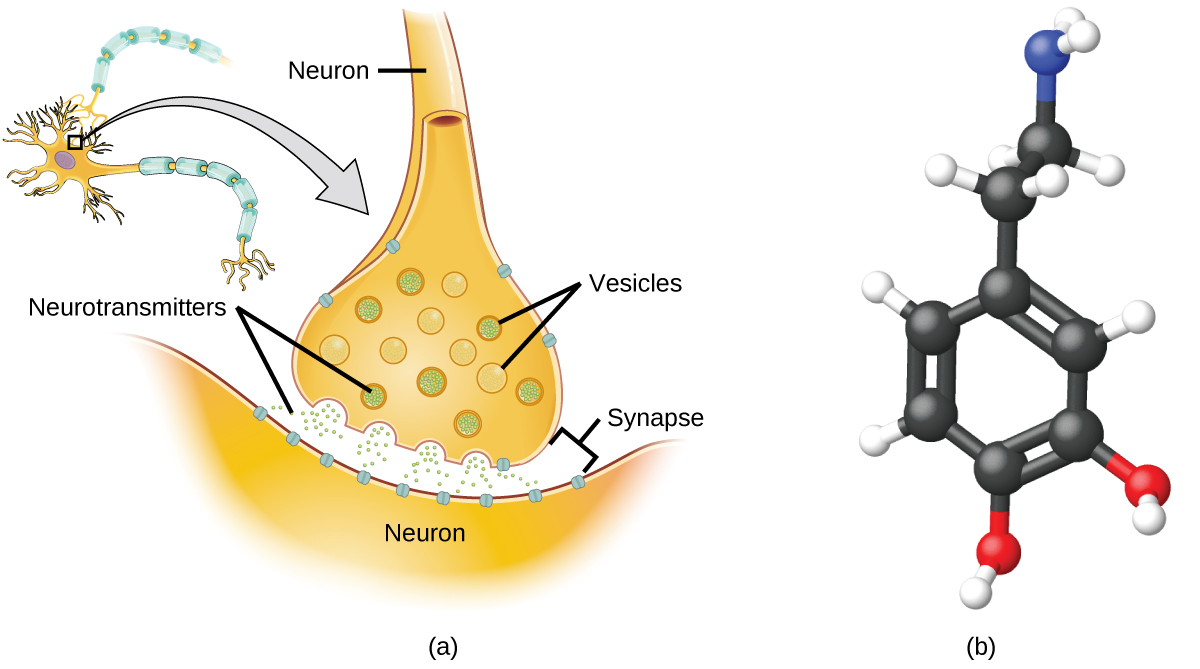
One important aspect of the complex processes related to dopamine signalling is the number of neurotransmitter molecules released during exocytosis. Since this number is a central factor in determining neurological response (and subsequent human thought and action), it is important to know how this number changes with certain controlled stimulations, such as the administration of drugs. It is also important to understand the mechanism responsible for any changes in the number of neurotransmitter molecules released—for example, some dysfunction in exocytosis, a change in the number of vesicles in the neuron, or a change in the number of neurotransmitter molecules in each vesicle.
Significant progress has been made recently in directly measuring the number of dopamine molecules stored in individual vesicles and the amount actually released when the vesicle undergoes exocytosis. Using miniaturized probes that can selectively detect dopamine molecules in very small amounts, scientists have determined that the vesicles of a certain type of mouse brain neuron contain an average of 30,000 dopamine molecules per vesicle (about 5 × 10−20 mol or 50 zmol). Analysis of these neurons from mice subjected to various drug therapies shows significant changes in the average number of dopamine molecules contained in individual vesicles, increasing or decreasing by up to three-fold, depending on the specific drug used. These studies also indicate that not all of the dopamine in a given vesicle is released during exocytosis, suggesting that it may be possible to regulate the fraction released using pharmaceutical therapies.[8]
Links to Interactive Learning Tools
Practice Molar Mass calculations from the Physics Classroom.
Practice Mole Conversions from the Physics Classroom.
Key Equations
[latex]n = \frac{m}{M}[/latex]
Number of atoms/ions/molecules = n x NA
Attribution & References
Except where otherwise noted, this page is adapted by Adrienne Richards from:
-
"6.1 Formula Mass and the Mole Concept" and "2.4 Counting Large Numbers of Atoms" In In General Chemistry 1 & 2 by Rice University, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson and is licensed under CC BY 4.0. Access for free at Chemistry (OpenStax)
- "3.1 Formula Mass and the Mole Concept" In Chemistry 2e (OpenStax) by Paul Flowers, Klaus Theopold, Richard Langley, & William R. Robinson, licensed under CC BY 4.0. Access for free at Chemistry 2e (OpenStax).
- 151.16 amu ; 151.16 g/mol ↵
- 310.18 amu ; 310.18 g/mol ↵
- 0.360 mol Be ↵
- 504.4 g Ar ↵
- 0.073 mol C12H22O11 ↵
- 14.2 g hydrazine ↵
- 4.586 x 1022 Au atoms ↵
- Omiatek, Donna M., Amanda J. Bressler, Ann-Sofie Cans, Anne M. Andrews, Michael L. Heien, and Andrew G. Ewing. “The Real Catecholamine Content of Secretory Vesicles in the CNS Revealed by Electrochemical Cytometry.” Scientific Report 3 (2013): 1447, accessed January 14, 2015, doi:10.1038/srep01447. ↵
sum of the average masses for all atoms represented in a chemical formula; for covalent compounds, this is also the molecular mass
the mass in grams of 1 mole of that substance

