12.2 Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law
Learning Objectives
By the end of this section, you will be able to:
- Identify the mathematical relationships between the various properties of gases
- Use the ideal gas law, and related gas laws, to compute the values of various gas properties under specified conditions
During the seventeenth and especially eighteenth centuries, driven both by a desire to understand nature and a quest to make balloons in which they could fly (Figure 12.2a), a number of scientists established the relationships between the macroscopic physical properties of gases, that is, pressure, volume, temperature, and amount of gas. Although their measurements were not precise by today’s standards, they were able to determine the mathematical relationships between pairs of these variables (e.g., pressure and temperature, pressure and volume) that hold for an ideal gas—a hypothetical construct that real gases approximate under certain conditions. Eventually, these individual laws were combined into a single equation—the ideal gas law—that relates gas quantities for gases and is quite accurate for low pressures and moderate temperatures. We will consider the key developments in individual relationships (for pedagogical reasons not quite in historical order), then put them together in the ideal gas law.
Pressure and Temperature: Gay-Lussac’s Law
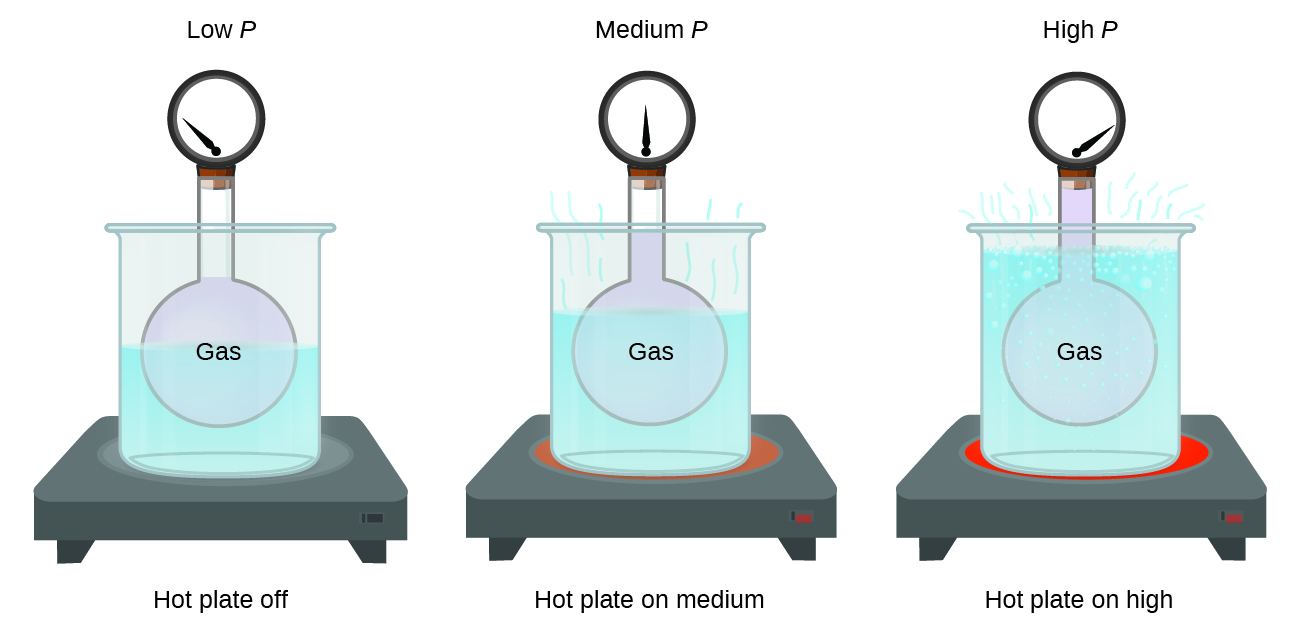
Imagine filling a rigid container attached to a pressure gauge with gas and then sealing the container so that no gas may escape. If the container is cooled, the gas inside likewise gets colder and its pressure is observed to decrease. Since the container is rigid and tightly sealed, both the volume and number of moles of gas remain constant. If we heat the sphere, the gas inside gets hotter (Figure 12.2b) and the pressure increases.
This relationship between temperature and pressure is observed for any sample of gas confined to a constant volume. An example of experimental pressure-temperature data is shown for a sample of air under these conditions in Figure 12.2c. We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then P and T are directly proportional (again, when volume and moles of gas are held constant); if the temperature on the kelvin scale increases by a certain factor, the gas pressure increases by the same factor.
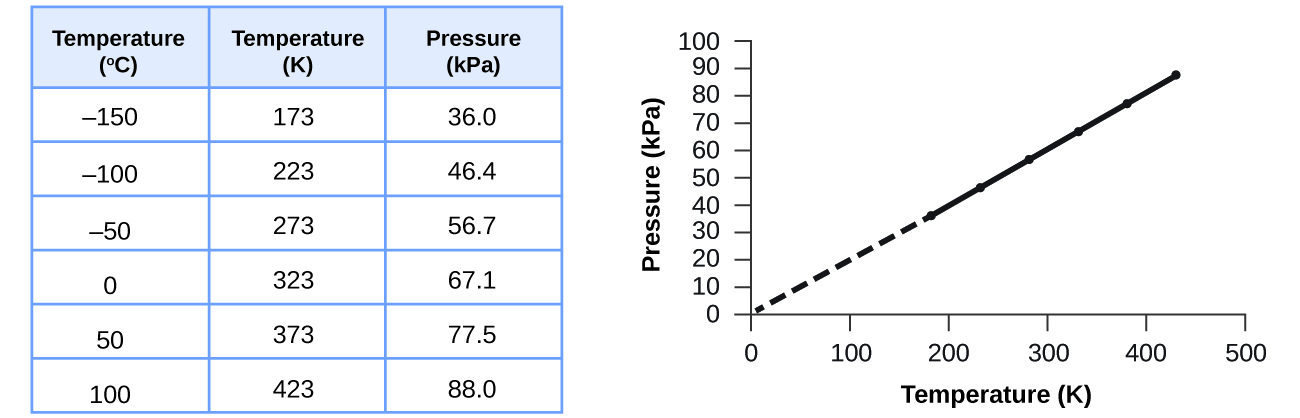
Guillaume Amontons was the first to empirically establish the relationship between the pressure and the temperature of a gas (~1700), and Joseph Louis Gay-Lussac determined the relationship more precisely (~1800). Because of this, the P-T relationship for gases is known as either Amontons’ law or Gay-Lussac’s law. Under either name, it states that the pressure of a given amount of gas is directly proportional to its temperature on the kelvin scale when the volume is held constant. Mathematically, this can be written:
where ∝ means “is proportional to,” and k is a proportionality constant that depends on the identity, amount, and volume of the gas.
For a confined, constant volume of gas, the ratio [latex]\frac{P}{T}[/latex] is therefore constant (i.e., [latex]\frac{P}{T} = k[/latex]). If the gas is initially in “Condition 1” (with P = P1 and T = T1), and then changes to “Condition 2” (with P = P2 and T = T2), we have that [latex]\frac{P_1}{T_1} = k[/latex] and [latex]\frac{P_2}{T_2} = k[/latex], which reduces to [latex]\frac{P_1}{T_1} = \frac{P_2}{T_2}[/latex]. This equation is useful for pressure-temperature calculations for a confined gas at constant volume. Note that temperatures must be on the kelvin scale for any gas law calculations (0 on the kelvin scale and the lowest possible temperature is called absolute zero). (Also note that there are at least three ways we can describe how the pressure of a gas changes as its temperature changes: We can use a table of values, a graph, or a mathematical equation.)
Example 12.2a
Predicting Change in Pressure with Temperature
A can of hair spray is used until it is empty except for the propellant, isobutane gas.
- On the can is the warning “Store only at temperatures below 120 °F (48.8 °C). Do not incinerate.” Why?
- The gas in the can is initially at 24 °C and 360 kPa, and the can has a volume of 350 mL. If the can is left in a car that reaches 50 °C on a hot day, what is the new pressure in the can?
Solution
A can of hair spray is used until it is empty except for the propellant, isobutane gas.
- The can contains an amount of isobutane gas at a constant volume, so if the temperature is increased by heating, the pressure will increase proportionately. High temperature could lead to high pressure, causing the can to burst. (Also, isobutane is combustible, so incineration could cause the can to explode.)
- We are looking for a pressure change due to a temperature change at constant volume, so we will use Amontons’/Gay-Lussac’s law. Taking P1 and T1 as the initial values, T2 as the temperature where the pressure is unknown and P2 as the unknown pressure, and converting °C to K, we have:
[latex]\frac{P_1}{T_1} = \frac{P_2}{T_2} \;\text{which means that} \;\frac{360 \;\text{kPa}}{297 \;\text{K}} = \frac{P_2}{323 \;\text{K}}[/latex]
Rearranging and solving gives: [latex]P_2 = \frac{360 \;\text{kPa} \times 323 \;\rule[0.25ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{K}}{297 \;\rule[0.25ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{K}} = 390 \;\text{kPa}[/latex]
Exercise 12.2a
A sample of nitrogen, N2, occupies 45.0 mL at 27 °C and 600 torr. What pressure will it have if cooled to –73 °C while the volume remains constant?
Check Your Answer[1]
Volume and Temperature: Charles’s Law
If we fill a balloon with air and seal it, the balloon contains a specific amount of air at atmospheric pressure, let’s say 1 atm. If we put the balloon in a refrigerator, the gas inside gets cold and the balloon shrinks (although both the amount of gas and its pressure remain constant). If we make the balloon very cold, it will shrink a great deal, and it expands again when it warms up.
Watch Liquid Nitrogen Experiments: The Balloon (3 mins)
Video source: Jefferson Lab. (2008, September 2). Liquid Nitrogen Experiments: The Balloon [Video]. YouTube.
These examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure are true in general: The volume increases as the temperature increases, and decreases as the temperature decreases. Volume-temperature data for a 1-mole sample of methane gas at 1 atm are listed and graphed in .
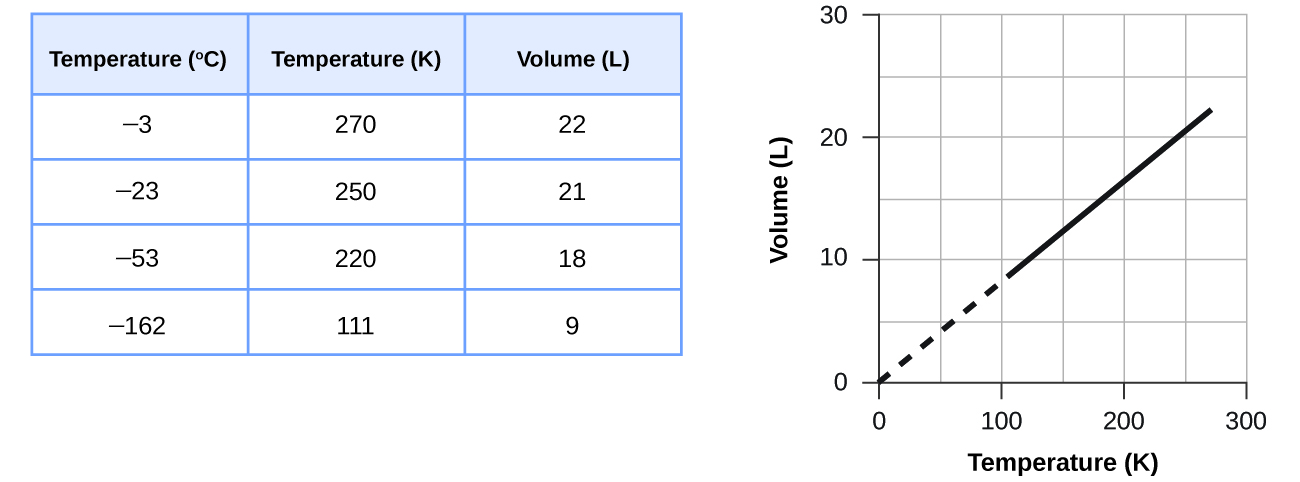
The relationship between the volume and temperature of a given amount of gas at constant pressure is known as Charles’ law in recognition of the French scientist and balloon flight pioneer Jacques Alexandre César Charles. Charles’ law states that the volume of a given amount of gas is directly proportional to its temperature on the kelvin scale when the pressure is held constant.
Mathematically, this can be written as:
with k being a proportionality constant that depends on the amount and pressure of the gas.
For a confined, constant pressure gas sample, [latex]\frac{V}{T}[/latex] is constant (i.e., the ratio = k), and as seen with the P-T relationship, this leads to another form of Charles’s law: [latex]\frac{V_1}{T_1} = \frac{V_2}{T_2}[/latex].
Example 12.2b
Predicting Change in Volume with Temperature
A sample of carbon dioxide, CO2, occupies 0.300 L at 10 °C and 750 torr. What volume will the gas have at 30 °C and 750 torr?
Solution
Because we are looking for the volume change caused by a temperature change at constant pressure, this is a job for Charles’s law. Taking V1 and T1 as the initial values, T2 as the temperature at which the volume is unknown and V2 as the unknown volume, and converting °C into K we have:
Rearranging and solving gives: [latex]V_2 = \frac{0.300 \;\text{L} \times 303 \;\rule[0.25ex]{0.8em}{0.1ex}\hspace{-0.8em}\text{K} }{283 \;\rule[0.25ex]{0.8em}{0.1ex}\hspace{-0.8em}\text{K}} = 0.321 \;\text{L}[/latex]
This answer supports our expectation from Charles’s law, namely, that raising the gas temperature (from 283 K to 303 K) at a constant pressure will yield an increase in its volume (from 0.300 L to 0.321 L).
Exercise 12.2b
A sample of oxygen, O2, occupies 32.2 mL at 30 °C and 452 torr. What volume will it occupy at –70 °C and the same pressure?
Check Your Answer[2]
Example 12.2c
Measuring Temperature with a Volume Change
Temperature is sometimes measured with a gas thermometer by observing the change in the volume of the gas as the temperature changes at constant pressure. The hydrogen in a particular hydrogen gas thermometer has a volume of 150.0 cm3 when immersed in a mixture of ice and water (0.00 °C). When immersed in boiling liquid ammonia, the volume of the hydrogen, at the same pressure, is 131.7 cm3. Find the temperature of boiling ammonia on the kelvin and Celsius scales.
Solution
A volume change caused by a temperature change at constant pressure means we should use Charles’s law. Taking V1 and T1 as the initial values, T2 as the temperature at which the volume is unknown and V2 as the unknown volume, and converting °C into K we have:
Rearrangement gives [latex]T_2 = \frac{131.7 \;\rule[0.25ex]{0.8em}{0.1ex}\hspace{-0.8em}\text{cm}^3 \times 273.15 \;\text{K} }{150.0 \;\rule[0.25ex]{0.8em}{0.1ex}\hspace{-0.8em}\text{cm}^3} = 239.8 \;\text{K}[/latex]
Subtracting 273.15 from 239.8 K, we find that the temperature of the boiling ammonia on the Celsius scale is –33.4 °C.
Exercise 12.2c
What is the volume of a sample of ethane at 467 K and 1.1 atm if it occupies 405 mL at 298 K and 1.1 atm?
Check Your Answer[3]
Volume and Pressure: Boyle’s Law
If we partially fill an airtight syringe with air, the syringe contains a specific amount of air at constant temperature, say 25 °C. If we slowly push in the plunger while keeping temperature constant, the gas in the syringe is compressed into a smaller volume and its pressure increases; if we pull out the plunger, the volume increases and the pressure decreases. This example of the effect of volume on the pressure of a given amount of a confined gas is true in general. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. In fact, if the volume increases by a certain factor, the pressure decreases by the same factor, and vice versa. Volume-pressure data for an air sample at room temperature are graphed in
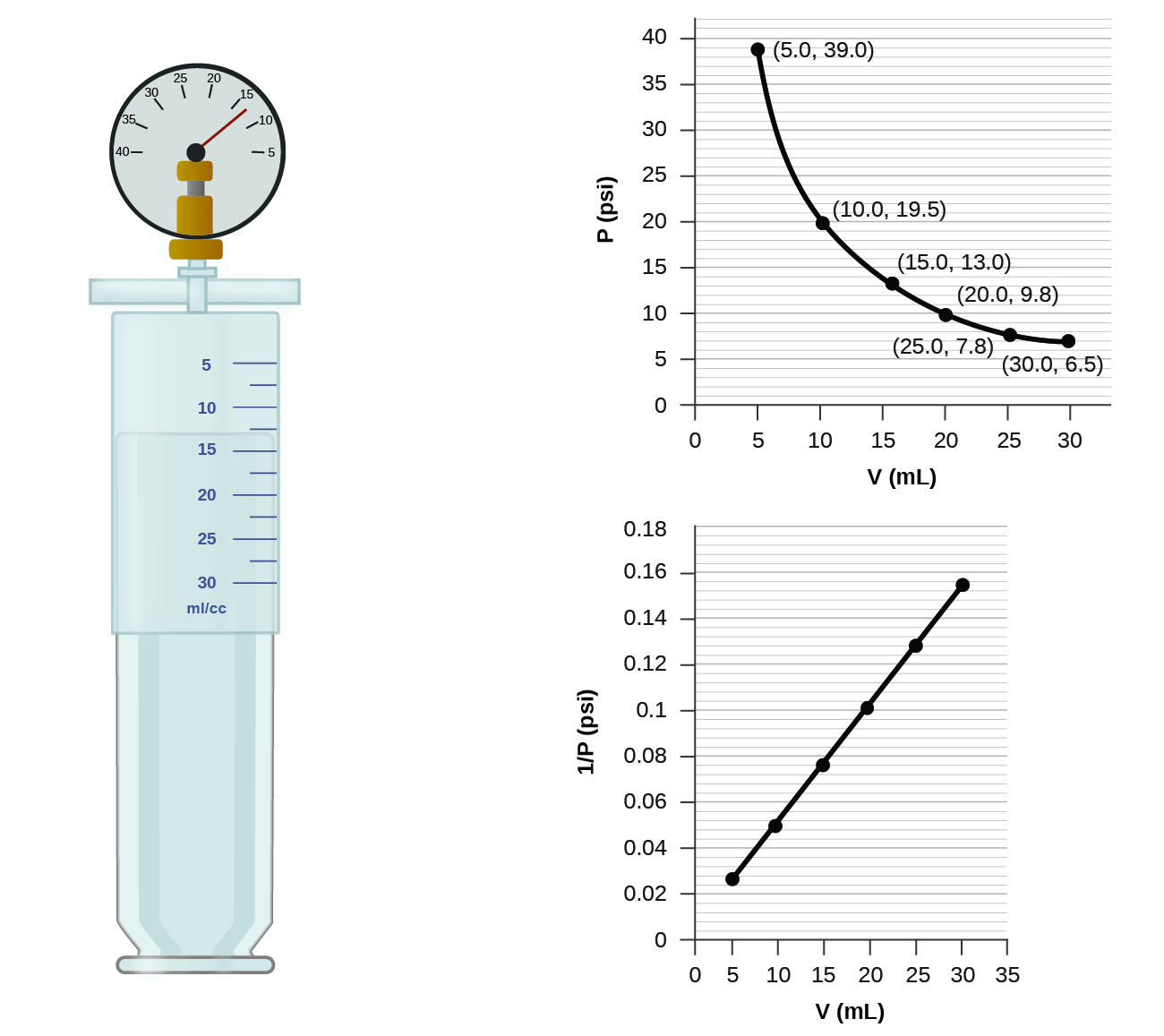
Unlike the P-T and V-T relationships, pressure and volume are not directly proportional to each other. Instead, P and V exhibit inverse proportionality: Increasing the pressure results in a decrease of the volume of the gas. Mathematically this can be written:
with k being a constant. Graphically, this relationship is shown by the straight line that results when plotting the inverse of the pressure ([latex]\frac{1}{P}[/latex]) versus the volume (V), or the inverse of volume ([latex]\frac{1}{V}[/latex]) versus the pressure (P). Graphs with curved lines are difficult to read accurately at low or high values of the variables, and they are more difficult to use in fitting theoretical equations and parameters to experimental data. For those reasons, scientists often try to find a way to “linearize” their data. If we plot P versus V, we obtain a hyperbola (see Figure 12.2f).
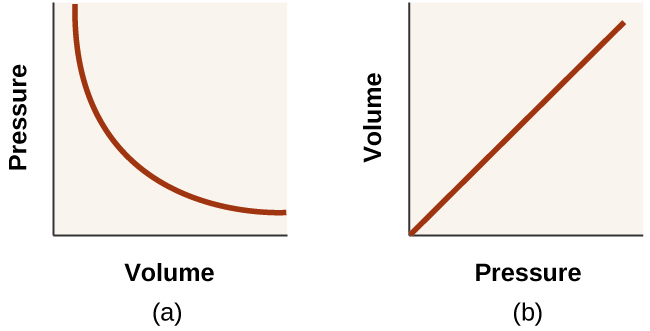
The relationship between the volume and pressure of a given amount of gas at constant temperature was first published by the English natural philosopher Robert Boyle over 300 years ago. It is summarized in the statement now known as Boyle’s law: The volume of a given amount of gas held at constant temperature is inversely proportional to the pressure under which it is measured.
Example 12.2d
Volume of a Gas Sample
The sample of gas in Figure 12.2e has a volume of 15.0 mL at a pressure of 13.0 psi. Determine the pressure of the gas at a volume of 7.5 mL, using:
- the P-V graph in Figure 12.2e
- the [latex]\frac{1}{p}[/latex] vs. V graph in Figure 12.2e
- the Boyle’s law equation
Comment on the likely accuracy of each method.
Solution
- Estimating from the P-V graph gives a value for P somewhere around 27 psi.
- Estimating from the [latex]\frac{1}{P}[/latex] versus V graph give a value of about 26 psi.
- From Boyle’s law, we know that the product of pressure and volume (PV) for a given sample of gas at a constant temperature is always equal to the same value. Therefore we have P1V1 = k and P2V2 = k which means that P1V1 = P2V2.
Using P1 and V1 as the known values 13.0 psi and 15.0 mL, P2 as the pressure at which the volume is unknown, and V2 as the unknown volume, we have:[latex]P_1 V_1 = P_2 V_2 \;\text{or} \; 13.0 \;\text{psi} \times 15.0 \;\text{mL} = P_2 \times 7.5 \;\text{mL}[/latex]Solving:[latex]P_2 = \frac{13.0 \;\text{psi} \times 15.0 \;\rule[0.5ex]{1.2em}{0.1ex}\hspace{-1.2em}\text{mL}}{7.5 \;\rule[0.5ex]{1.2em}{0.1ex}\hspace{-1.2em}\text{mL}} = 26 \;\text{psi}[/latex]
It was more difficult to estimate well from the P-V graph, so (a) is likely more inaccurate than (b) or (c). The calculation will be as accurate as the equation and measurements allow.
Exercise 12.2d
The sample of gas in Figure 12.2e has a volume of 30.0 mL at a pressure of 6.5 psi. Determine the volume of the gas at a pressure of 11.0 psi, using:
- the P-V graph in Figure 12.2e
- the [latex]\frac{1}{P}[/latex] vs. V graph in Figure 12.2e
- the Boyle’s law equation
Comment on the likely accuracy of each method.
Check Your Answer[4]
Breathing and Boyle’s Law
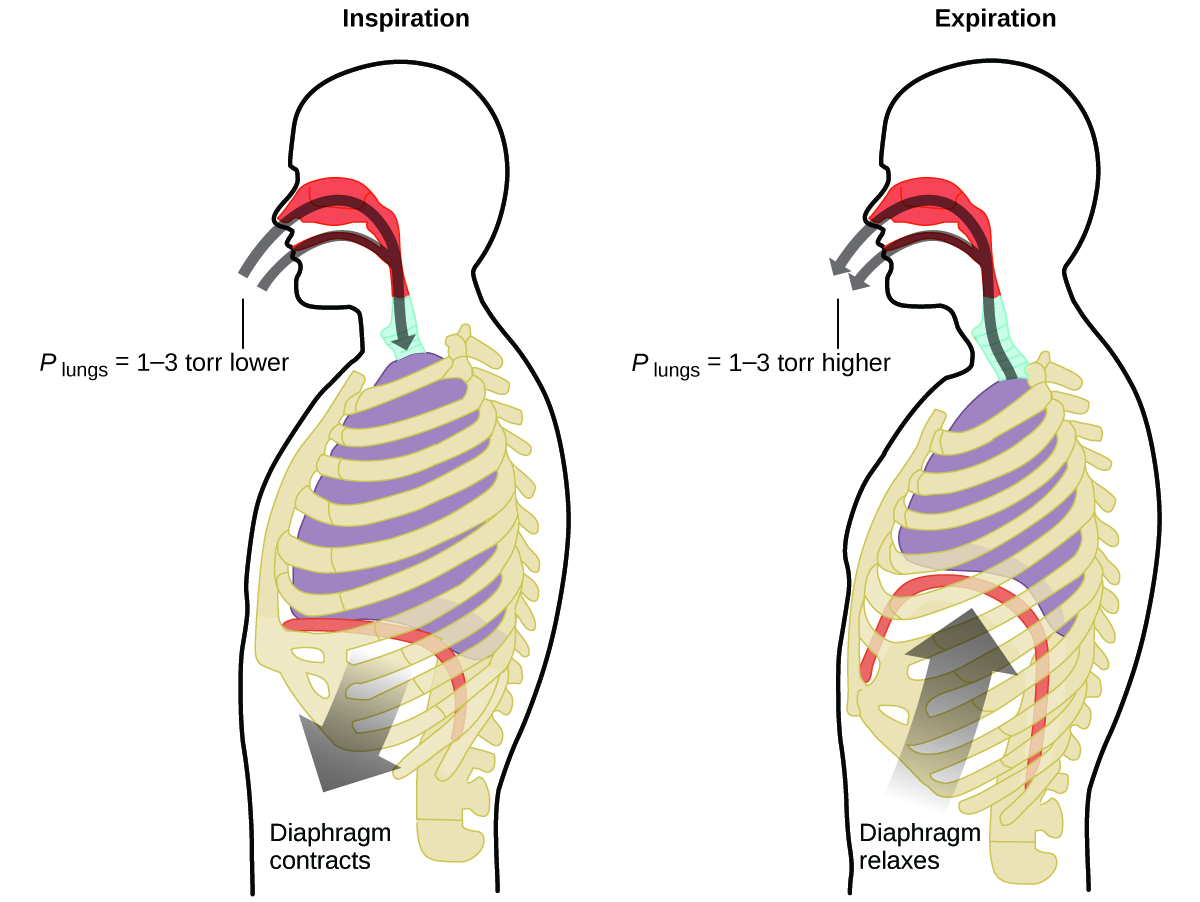
What do you do about 20 times per minute for your whole life, without break, and often without even being aware of it? The answer, of course, is respiration, or breathing. How does it work? It turns out that the gas laws apply here. Your lungs take in gas that your body needs (oxygen) and get rid of waste gas (carbon dioxide). Lungs are made of spongy, stretchy tissue that expands and contracts while you breathe. When you inhale, your diaphragm and intercostal muscles (the muscles between your ribs) contract, expanding your chest cavity and making your lung volume larger. The increase in volume leads to a decrease in pressure (Boyle’s law). This causes air to flow into the lungs (from high pressure to low pressure). When you exhale, the process reverses: Your diaphragm and rib muscles relax, your chest cavity contracts, and your lung volume decreases, causing the pressure to increase (Boyle’s law again), and air flows out of the lungs (from high pressure to low pressure). You then breathe in and out again, and again, repeating this Boyle’s law cycle for the rest of your life (Figure 12.2g).
Moles of Gas and Volume: Avogadro’s Law
The Italian scientist Amedeo Avogadro advanced a hypothesis in 1811 to account for the behaviour of gases, stating that equal volumes of all gases, measured under the same conditions of temperature and pressure, contain the same number of molecules. Over time, this relationship was supported by many experimental observations as expressed by Avogadro’s law: For a confined gas, the volume (V) and number of moles (n) are directly proportional if the pressure and temperature both remain constant.
In equation form, this is written as:
Mathematical relationships can also be determined for the other variable pairs, such as P versus n, and n versus T.
Exercise 12.2e
Practice using the following PhET simulation: Gas Properties
The Ideal Gas Law
To this point, four separate laws have been discussed that relate pressure, volume, temperature, and the number of moles of the gas:
- Boyle’s law: PV = constant at constant T and n
- Amontons’ law: [latex]\frac{P}{T}[/latex] = constant at constant V and n
- Charles’ law: [latex]\frac{V}{T}[/latex] = constant at constant P and n
- Avogadro’s law: [latex]\frac{V}{n}[/latex] = constant at constant P and T
Combining these four laws yields the ideal gas law, a relation between the pressure, volume, temperature, and number of moles of a gas:
where P is the pressure of a gas, V is its volume, n is the number of moles of the gas, T is its temperature on the kelvin scale, and R is a constant called the ideal gas constant or the universal gas constant. The units used to express pressure, volume, and temperature will determine the proper form of the gas constant as required by dimensional analysis, the most commonly encountered values being 0.08206 L atm mol–1 K–1 and 8.314 kPa L mol–1 K–1.
Gases whose properties of P, V, and T are accurately described by the ideal gas law (or the other gas laws) are said to exhibit ideal behaviour or to approximate the traits of an ideal gas. An ideal gas is a hypothetical construct that may be used along with kinetic molecular theory to effectively explain the gas laws as will be described in a later module of this chapter. Although all the calculations presented in this module assume ideal behaviour, this assumption is only reasonable for gases under conditions of relatively low pressure and high temperature. In the final module of this chapter, a modified gas law will be introduced that accounts for the non-ideal behaviour observed for many gases at relatively high pressures and low temperatures.
The ideal gas equation contains five terms, the gas constant R and the variable properties P, V, n, and T. Specifying any four of these terms will permit use of the ideal gas law to calculate the fifth term as demonstrated in the following example exercises.
Example 12.2e
Using the Ideal Gas Law
Methane, CH4, is being considered for use as an alternative automotive fuel to replace gasoline. One gallon of gasoline could be replaced by 655 g of CH4. What is the volume of this much methane at 25 °C and 745 torr?
Solution
We must rearrange PV = nRT to solve for V: [latex]V = \frac{nRT}{P}[/latex]
If we choose to use R = 0.08206 L atm mol–1 K–1, then the amount must be in moles, temperature must be in kelvin, and pressure must be in atm.
Converting into the “right” units:
It would require 1020 L (269 gal) of gaseous methane at about 1 atm of pressure to replace 1 gal of gasoline. It requires a large container to hold enough methane at 1 atm to replace several gallons of gasoline.
Exercise 12.2f
Calculate the pressure in bar of 2520 moles of hydrogen gas stored at 27 °C in the 180-L storage tank of a modern hydrogen-powered car.
Check Your Answer[5]
If the number of moles of an ideal gas are kept constant under two different sets of conditions, a useful mathematical relationship called the combined gas law is obtained: [latex]\frac{P_1 V_1}{T_1} = \frac{P_2 V_2}{T_2}[/latex] using units of atm, L, and K. Both sets of conditions are equal to the product of n ×R (where n = the number of moles of the gas and R is the ideal gas law
Example 12.2f
Using the Combined Gas Law
When filled with air, a typical scuba tank with a volume of 13.2 L has a pressure of 153 atm (Figure 12.2h). If the water temperature is 27 °C, how many litres of air will such a tank provide to a diver’s lungs at a depth of approximately 70 feet in the ocean where the pressure is 3.13 atm?

Solution
Letting 1 represent the air in the scuba tank and 2 represent the air in the lungs, and noting that body temperature (the temperature the air will be in the lungs) is 37 °C, we have:
[latex]\frac{P_1 V_1}{T_1} = \frac{P_2 V_2}{T_2} \longrightarrow \frac{(153 \;\text{atm})(13.2 \;\text{L})}{(300 \;\text{K})} = \frac{(3.13 \;\text{atm})(V_2)}{(310 \;\text{K})}[/latex]
Solving for V2:
(Note: Be advised that this particular example is one in which the assumption of ideal gas behaviour is not very reasonable, since it involves gases at relatively high pressures and low temperatures. Despite this limitation, the calculated volume can be viewed as a good “ballpark” estimate.)
Exercise 12.2g
A sample of ammonia is found to occupy 0.250 L under laboratory conditions of 27 °C and 0.850 atm. Find the volume of this sample at 0 °C and 1.00 atm.
Check Your Answer[6]
The Interdependence between Ocean Depth and Pressure in Scuba Diving
Whether scuba diving at the Great Barrier Reef in Australia (shown in Figure 12.2i) or in the Caribbean, divers must understand how pressure affects a number of issues related to their comfort and safety.

Pressure increases with ocean depth, and the pressure changes most rapidly as divers reach the surface. The pressure a diver experiences is the sum of all pressures above the diver (from the water and the air). Most pressure measurements are given in units of atmospheres, expressed as “atmospheres absolute” or ATA in the diving community: Every 33 feet of salt water represents 1 ATA of pressure in addition to 1 ATA of pressure from the atmosphere at sea level. As a diver descends, the increase in pressure causes the body’s air pockets in the ears and lungs to compress; on the ascent, the decrease in pressure causes these air pockets to expand, potentially rupturing eardrums or bursting the lungs. Divers must therefore undergo equalization by adding air to body airspaces on the descent by breathing normally and adding air to the mask by breathing out of the nose or adding air to the ears and sinuses by equalization techniques; the corollary is also true on ascent, divers must release air from the body to maintain equalization. Buoyancy, or the ability to control whether a diver sinks or floats, is controlled by the buoyancy compensator (BCD). If a diver is ascending, the air in his BCD expands because of lower pressure according to Boyle’s law (decreasing the pressure of gases increases the volume). The expanding air increases the buoyancy of the diver, and she or he begins to ascend. The diver must vent air from the BCD or risk an uncontrolled ascent that could rupture the lungs. In descending, the increased pressure causes the air in the BCD to compress and the diver sinks much more quickly; the diver must add air to the BCD or risk an uncontrolled descent, facing much higher pressures near the ocean floor. The pressure also impacts how long a diver can stay underwater before ascending. The deeper a diver dives, the more compressed the air that is breathed because of increased pressure: If a diver dives 33 feet, the pressure is 2 ATA and the air would be compressed to one-half of its original volume. The diver uses up available air twice as fast as at the surface.
Watch The ABC's of gas: Avogadro, Boyle, Charles - Brian Bennett
Video source: TED-Ed. (2012c, October 9). The ABC’s of gas: Avogadro, Boyle, Charles - Brian Bennett [Video]. YouTube. https://www.youtube.com/watch?v=BY9VGS2eXas
Standard Conditions of Temperature and Pressure
We have seen that the volume of a given quantity of gas and the number of molecules (moles) in a given volume of gas vary with changes in pressure and temperature. Chemists sometimes make comparisons against a standard temperature and pressure (STP) for reporting properties of gases: 273.15 K and 1 atm (101.325 kPa). At STP, an ideal gas has a volume of about 22.4 L—this is referred to as the standard molar volume (Figure 12.2j).
Exercise 12.2h
Check Your Learning Exercise (Text Version)
A spray can is used until it is empty except for the propellant gas, which has a pressure of 1344 torr at 23 °C. If the can is thrown into a fire (T = 475 °C), what will be the pressure in the hot can?
Check Your Answer[7]
Source: "Exercise 12.2h" is adapted from "Exercise 8.2-10" in General Chemistry 1 & 2, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson, licensed under CC BY 4.0.
Links to Interactive Learning Tools
Explore Volume-Temperature Gas Law from the Physics Classroom.
Explore Pressure-Volume Gas Law from the Physics Classroom.
Explore Pressure-Temperature Gas Law from the Physics Classroom.
Key Equations
- PV = nRT
Attribution & References
(also, Gay-Lussac’s law) pressure of a given number of moles of gas is directly proportional to its kelvin temperature when the volume is held constant
temperature at which the volume of a gas would be zero according to Charles’s law.
volume of a given number of moles of gas is directly proportional to its kelvin temperature when the pressure is held constant
volume of a given number of moles of gas held at constant temperature is inversely proportional to the pressure under which it is measured
volume of a gas at constant temperature and pressure is proportional to the number of gas molecules
relation between the pressure, volume, amount, and temperature of a gas under conditions derived by combination of the simple gas laws
constant derived from the ideal gas equation R = 0.08226 L atm mol–1 K–1 or 8.314 L kPa mol–1 K–1
hypothetical gas whose physical properties are perfectly described by the gas laws
273.15 K (0 °C) and 1 atm (101.325 kPa)
volume of 1 mole of gas at STP, approximately 22.4 L for gases behaving ideally

