5.1 Early Atomic Theory: Dalton’s Model of the Atom
Learning Objectives
By the end of this section, you will be able to:
- Summarize the postulates of Dalton’s atomic theory
- Apply Dalton’s atomic theory to explain the laws of definite and multiple proportions
The language used in chemistry is seen and heard in many disciplines, ranging from medicine to engineering to forensics to art. The language of chemistry includes its own vocabulary as well as its own form of shorthand. Chemical symbols are used to represent atoms and elements. Chemical formulas depict molecules as well as the composition of compounds. Chemical equations provide information about the quality and quantity of the changes associated with chemical reactions.
This chapter will lay the foundation for our study of the language of chemistry. The concepts of this foundation include the atomic theory, the composition and mass of an atom, and the variability of the composition of isotopes.
Atomic Theory through the Nineteenth Century
Watch The 2,400-year search for the atom - Theresa Doud (6 mins)
Video source: TED-Ed. (2014, December 8). The 2,400-year search for the atom - Theresa Doud [Video]. YouTube.
The earliest recorded discussion of the basic structure of matter comes from ancient Greek philosophers, the scientists of their day. In the fifth century BC, Leucippus and Democritus argued that all matter was composed of small, finite particles that they called atomos, a term derived from the Greek word for “indivisible.” They thought of atoms as moving particles that differed in shape and size, and which could join together. Later, Aristotle and others came to the conclusion that matter consisted of various combinations of the four “elements”—fire, earth, air, and water—and could be infinitely divided. Interestingly, these philosophers thought about atoms and “elements” as philosophical concepts, but apparently never considered performing experiments to test their ideas.
The Aristotelian view of the composition of matter held sway for over two thousand years, until English schoolteacher John Dalton helped to revolutionize chemistry with his hypothesis that the behaviour of matter could be explained using an atomic theory. First published in 1807, many of Dalton’s hypotheses about the microscopic features of matter are still valid in modern atomic theory. Here are the postulates of Dalton’s atomic theory.
- Matter is composed of exceedingly small, indivisible particles called atoms. An atom is the smallest unit of an element that can participate in a chemical change.
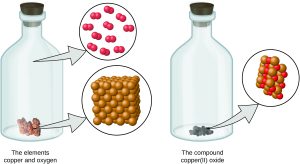
- An element consists of only one type of atom, which has a mass that is characteristic of the element and is the same for all atoms of that element (Figure 5.1a). A macroscopic sample of an element contains an incredibly large number of atoms, all of which have identical chemical properties

Figure 5.1a Macroscopic vs Microscopic Visual the Element Copper: A pre-1982 copper penny (left) contains approximately 3 × 1022 copper atoms (several dozen are represented as brown spheres at the right), each of which has the same chemical properties. (credit: modification of work by slgc, CC BY 2.0; in Chemistry (OpenStax), CC BY 4.0). - Atoms of one element differ in properties from atoms of all other elements.
- A compound consists of atoms of two or more elements combined in a small, whole-number ratio. In a given compound, the numbers of atoms of each of its elements are always present in the same ratio (Figure 5.1b).

Figure 5.1b Macroscopic vs Microscopic Visual of the Compound Copper(II) Oxide: Copper(II) oxide, a powdery, black compound, results from the combination of two types of atoms—copper (brown, larger spheres) and oxygen (red, smaller spheres)—in a 1:1 ratio. (credit: modification of work by Chemicalinterest, PD; in Chemistry (OpenStax), CC BY 4.0). - Atoms are neither created nor destroyed during a chemical change, but are instead rearranged and regrouped to yield substances that are different from those present before the change (Figure 5.1c).
Dalton's atomic theory laid the foundation in the development of chemistry. Most of his postulates remain valid; however, some of his conclusions have been revolutionized because further investigations have shown that
- Atoms are composed of subatomic particles; they are not indivisible
- Not all the atoms of a specific element have the exact same mass; an element can exist in different forms, called isotopes
- Atoms, under special conditions, can be decomposed.
Source: (Hein & Arena, 2014, p. 83)
Despite these flaws, Dalton's atomic theory provides a microscopic explanation of the many macroscopic properties of matter that you’ve learned about. For example, if an element such as copper consists of only one kind of atom, then it cannot be broken down into simpler substances, that is, into substances composed of fewer types of atoms. And if atoms are neither created nor destroyed during a chemical change, then the total mass of matter present when matter changes from one type to another will remain constant (the law of conservation of matter).
Example 5.1a
Testing Dalton’s Atomic Theory
In the following drawing, the green, larger spheres represent atoms of a certain element. The blue, smaller spheres represent atoms of another element. If the spheres touch, they are part of a single unit of a compound. Does the following chemical change represented by these symbols violate any of the ideas of Dalton’s atomic theory? If so, which one?
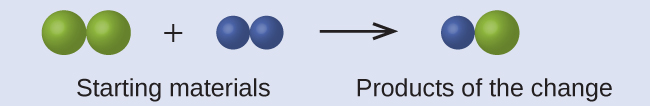
Solution
The starting materials consist of two green, larger spheres and two blue, smaller spheres. The products consist of only one green sphere and one blue sphere. This violates Dalton’s postulate that atoms are neither created nor destroyed during a chemical change, but are merely redistributed. (In this case, atoms appear to have been destroyed.)
Exercise 5.1a
In the following drawing, the green, larger spheres represent atoms of a certain element. The blue, smaller spheres represent atoms of another element. If the spheres touch, they are part of a single unit of a compound. Does the following chemical change represented by these symbols violate any of the ideas of Dalton’s atomic theory? If so, which one?
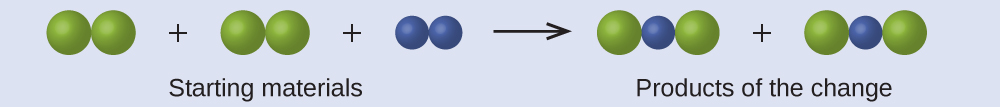
Check Your Answer
Exercise 5.1b
Check Your Learning Exercise (Text Version)
Choose the answer that best answers the questions for each of the multiple choice questions.
- According to Dalton’s Atomic Theory, matter consists of indivisible
- Molecules
- Cells
- Atoms
- Subatomic particles
- Dalton’s Atomic Theory postulates mass is neither created nor destroyed in a chemical reaction. This supports the
- Law of constant proportions
- Law of conservation of mass
- Law of multiple proportions
- Law of electric force
- Fill in the blanks with the correct pair of terms from the choices below according to Dalton’s Atomic Theory: All atoms of a given element have identical ________ including identical _______.
- Temperature, volume
- Force, pressure
- Weight, volume
- Physical and chemical properties, mass
- Which of the following is NOT a postulate of Dalton’s Atomic Theory?
- Atoms that combine to form new molecules do so in simple, whole number ratios
- A chemical reaction is a rearrangement of atoms. No atoms are created or destroyed.
- All elements are composed of small particles called atoms.
- Atoms of a given element are always identical.
- Atoms are always on motion
- 5. Most of Dalton’s postulates remain valid; however, some of Dalton’s atomic theory postulates have been proven to be false. Which of the following postulates were determined to be incorrect?
- Matter is composed of exceedingly small, indivisible particles.
- Elements consist of only one type of identical atom, which has the same mass for all atoms.
- Theories stated in a) and b) were both proven to be false
- Neither theory stated in a) nor b) were proven to be false
Check Your Answer
Source: "Exercise 5.1b" by Jackie MacDonald, licensed under CC BY-NC-SA 4.0
Dalton knew of the experiments of French chemist Joseph Proust, who demonstrated that all samples of a pure compound contain the same elements in the same proportion by mass. This statement is known as the law of definite proportions or the law of constant composition. The suggestion that the numbers of atoms of the elements in a given compound always exist in the same ratio is consistent with these observations. For example, when different samples of isooctane (a component of gasoline and one of the standards used in the octane rating system) are analyzed, they are found to have a carbon-to-hydrogen mass ratio of 5.33:1, as shown in Table 5.1a.
| Sample | Carbon | Hydrogen | Mass Ratio |
|---|---|---|---|
| A | 14.82 g | 2.78 g | [latex]\frac{14.82 \text{g carbon}}{2.78 \text{g hydrogen}} = \frac{5.33 \text{g carbon}}{1.00 \text{g hydrogen}}[/latex] |
| B | 22.33 g | 4.19 g | [latex]\frac{22.33 \text{g carbon}}{4.19 \text{g hydrogen}} = \frac{5.33 \text{g carbon}}{1.00 \text{g hydrogen}}[/latex] |
| C | 19.40 g | 3.64 g | [latex]\frac{19.40 \text{g carbon}}{3.64 \text{g hydrogen}} = \frac{5.33 \text{g carbon}}{1.00 \text{g hydrogen}}[/latex] |
It is worth noting that although all samples of a particular compound have the same mass ratio, the converse is not true in general. That is, samples that have the same mass ratio are not necessarily the same substance. For example, there are many compounds other than isooctane that also have a carbon-to-hydrogen mass ratio of 5.33 : 1.00.
Watch Thinking Reeds Chemistry - The Law of Definite Proportions (4 mins 13 sec)
Video Source: Thinking Reeds Chemistry (2016, September 6). Thinking Reeds Chemistry - The Law of Definite Proportions [Video]. YouTube.
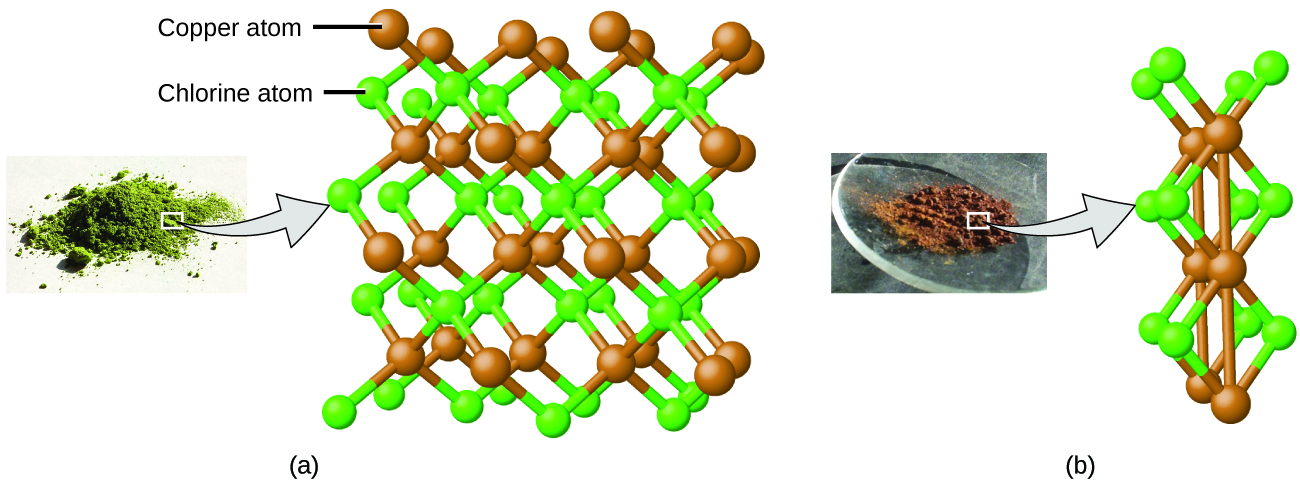
Dalton also used data from Proust, as well as results from his own experiments, to formulate another interesting law. The law of multiple proportions states that when two elements react to form more than one compound, a fixed mass of one element will react with masses of the other element in a ratio of small, whole numbers. For example, copper and chlorine can form a green, crystalline solid with a mass ratio of 0.558 g chlorine to 1 g copper, as well as a brown crystalline solid with a mass ratio of 1.116 g chlorine to 1 g copper. These ratios by themselves may not seem particularly interesting or informative; however, if we take a ratio of these ratios, we obtain a useful and possibly surprising result: a small, whole-number ratio.
This 2-to-1 ratio means that the brown compound has twice the amount of chlorine per amount of copper as the green compound. When referencing Figure 5.1d, the above can be explained by atomic theory if the copper-to-chlorine ratio in the brown compound (Figure 5.1d (b)) is 1 copper atom to 2 chlorine atoms, and the ratio in the green compound (Figure 5.1d (a)) is 1 copper atom to 1 chlorine atom. The ratio of chlorine atoms in compound B compared to compound A (and thus the ratio of their masses) is therefore 2 to 1 (Figure 5.1d).
Watch Multiple Proportions (1 min 11 sec)
Video Source: kwesiamoa. (2010, January 20). Multiple Proportions [Video]. YouTube.
Example 5.1b
Laws of Definite and Multiple Proportions
A sample of compound A (a clear, colourless gas) is analyzed and found to contain 4.27 g carbon and 5.69 g oxygen. A sample of compound B (also a clear, colourless gas) is analyzed and found to contain 5.19 g carbon and 13.84 g oxygen. Are these data an example of the law of definite proportions, the law of multiple proportions, or neither? What do these data tell you about substances A and B?
Solution
In compound A, the mass ratio of carbon to oxygen is:
In compound B, the mass ratio of carbon to oxygen is:
The ratio of these ratios is:
The ratio of oxygen atoms of compound A to compound B (and thus the ratio of their masses) is 1 to 2. This supports the law of multiple proportions. This means that A and B are different compounds, with A having one-half as much oxygen per amount of carbon as compound B. A possible pair of compounds that would fit this relationship would be A = CO and B = CO2.
Exercise 5.1c
A sample of compound X (a clear, colourless, combustible liquid with a noticeable odour) is analyzed and found to contain 14.13 g carbon and 2.96 g hydrogen. A sample of compound Y (a clear, colourless, combustible liquid with a noticeable odour that is slightly different from X’s odour) is analyzed and found to contain 19.91 g carbon and 3.34 g hydrogen. Are these data an example of the law of definite proportions, the law of multiple proportions, or neither? What do these data tell you about substances X and Y?
Check Your Answer
Links to Interactive Learning Tools
Explore the Timeline of Atomic Discovery from eCampusOntario H5P Studio.
Attribution & References
Except where otherwise noted, this page is adapted by Jackie MacDonald from “2.1 Early ideas in atomic theory” In Chemistry 2e (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson is licensed under CC BY 4.0. Access for free at Chemistry 2e (Open Stax).
References
Hein, M., & Arena, S. (2014). Foundations of College Chemistry (14th edition). Wiley & Sons.
- The starting materials consist of four green, larger spheres and two blue, smaller spheres. The products consist of four green, larger spheres and two blue, smaller spheres. This does not violate any of Dalton’s postulates: Atoms are neither created nor destroyed, but are redistributed in small, whole-number ratios. ↵
- 1c; 2b; 3d; 4e; 5c ↵
- In compound X, the mass ratio of carbon to hydrogen is [latex]\frac{14.13 \text{g C}}{2.96 \text{g H}}[/latex]. In compound Y, the mass ratio of carbon to oxygen is [latex]\frac{19.91 \text{g C}}{3.34 \text{g H}}[/latex]. The ratio of these ratios is [latex]\frac{\frac{14.13 \text{g C}}{2.96 \text{g H}}}{\frac{19.91 \text{g C}}{3.34 \text{g H}}} = \frac{4.77 \text{g C/g H}}{5.96 \text{g C/g H}} = 0.800 = \frac{4}{5}[/latex]. This small, whole-number ratio supports the law of multiple proportions. This means that X and Y are different compounds. ↵
set of postulates that established the fundamental properties of atoms
smallest particle of an element that can enter into a chemical combination
substance that is composed of a single type of atom; a substance that cannot be decomposed by a chemical change
(also, law of constant composition) all samples of a pure compound contain the same elements in the same proportions by mass
(also, law of definite proportions) all samples of a pure compound contain the same elements in the same proportions by mass
when two elements react to form more than one compound, a fixed mass of one element will react with masses of the other element in a ratio of small whole numbers

