Chapter 5 – Review
5.1 Early Atomic Theory: Dalton's Model of the Atom
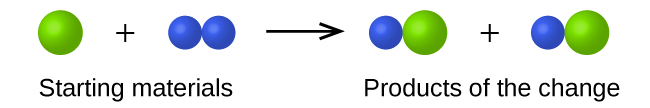
- In the following drawing, the green, larger spheres represent atoms of a certain element. The blue, smaller spheres represent atoms of another element. If the spheres of different elements touch, they are part of a single unit of a compound. The following chemical change represented by these spheres may violate one of the ideas of Dalton’s atomic theory. Which one?
 Check Answer: [1]
Check Answer: [1] - Which postulate of Dalton’s theory is consistent with the following observation concerning the weights of reactants and products? When 100 grams of solid calcium carbonate is heated, 44 grams of carbon dioxide and 56 grams of calcium oxide are produced.
Check Answer: [2] - Identify the postulate of Dalton’s theory that is violated by the following observations: 59.95% of one sample of titanium dioxide is titanium; 60.10% of a different sample of titanium dioxide is titanium. Check Answer: [3]
- Samples of compound X, Y, and Z are analyzed, with results shown in the data table below.
Qualitative and Quantitative Analysis of Compounds X, Y, Z Compound Description Mass of Carbon Mass of Hydrogen X clear, colourless, liquid with strong odour 1.776 g 0.148 g Y clear, colourless, liquid with strong odour 1.974 g 0.329 g Z clear, colourless, liquid with strong odour 7.812 g 0.651 g Do these data provide example(s) of the law of definite proportions, the law of multiple proportions, neither, or both? What do these data tell you about compounds X, Y, and Z? Check Answer: [4]
- For the following questions, state whether the statement regarding Dalton's Atomic Theory is True or False. If it is false, provide the correct word or phrase for the bolded term to make that statement true.
- According to Dalton’s Atomic Theory, matter consists of indivisible particles called atoms.
- Dalton’s Atomic Theory postulates mass is neither created nor destroyed in a chemical reaction. This supports the law of constant proportions.
- According to Dalton's atomic theory, all atoms of a given element have identical properties including identical mass.
- Two of Dalton's postulates include (1) matter is composed of exceedingly small, indivisible particles and (2) elements consist of only one type of identical atom, which has the same mass for all atoms. These two theories have been proven to be valid theories by later scientists.
Check Answer: [5]
5.2 Electric Charge
- For the following questions, state whether the statement regarding electric charge is True or False. If it is false, provide the correct word or phrase for the bolded term to make that statement true.
- Electric charges can be positive and negative.
- Electric force can be either be equal or repulsive.
- If two interacting objects carry opposite charges, it results in electrostatic repulsion.
- A balloon is rubbed on hair to gain charge. Then the comb is placed near running water from a tap. You observe that the stream of water is bending towards the comb. You conclude that the comb and water must have similar charges.
Check Answer: [6]
- Does the force of attraction increase, decrease, or stay the same as the distance decreases between two oppositely charged objects (as the objects near one another)?
Check Answer: [7] - Does the force of attraction increase, decrease, or stay the same as the distance increases between two oppositely charged objects (as the objects get further from one another)?
Check Answer: [8] - Explain what is meant by induction in reference to electric forces.
Check Answer: [9]
5.3 Subatomic Particles of the Atom
- How are electrons and protons similar? How are they different?
Check Answer: [10] - How are protons and neutrons similar? How are they different?
- Check Answer: [11]
- Predict and test the behaviour of α particles fired at a “plum pudding” model atom.
- Predict the paths taken by α particles that are fired at atoms with a Thomson’s plum pudding model structure. Explain why you expect the α particles to take these paths.
- If α particles of higher energy than those in (a) are fired at plum pudding atoms, predict how their paths will differ from the lower-energy α particle paths. Explain your reasoning.
- Now test your predictions from (a) and (b). Open the Rutherford Scattering simulation and select the “Plum Pudding Atom” tab. Set “Alpha Particles Energy” to “min,” and select “show traces.” Click on the gun to start firing α particles. Does this match your prediction from (a)? If not, explain why the actual path would be that shown in the simulation. Hit the pause button, or “Reset All.” Set “Alpha Particles Energy” to “max,” and start firing α particles. Does this match your prediction from (b)? If not, explain the effect of increased energy on the actual paths as shown in the simulation.
Check Answer: [12]
- Predict and test the behaviour of α particles fired at a Rutherford atom model.
- Predict the paths taken by α particles that are fired at atoms with a Rutherford atom model structure. Explain why you expect the α particles to take these paths.
- If α particles of higher energy than those in (a) are fired at Rutherford atoms, predict how their paths will differ from the lower-energy α particle paths. Explain your reasoning.
- Predict how the paths taken by the α particles will differ if they are fired at Rutherford atoms of elements other than gold. What factor do you expect to cause this difference in paths, and why?
- Now test your predictions from (a), (b), and (c). Open the Rutherford Scattering simulation and select the “Rutherford Atom” tab. Due to the scale of the simulation, it is best to start with a small nucleus, so select “20” for both protons and neutrons, “min” for energy, show traces, and then start firing α particles. Does this match your prediction from (a)? If not, explain why the actual path would be that shown in the simulation. Pause or reset, set energy to “max,” and start firing α particles. Does this match your prediction from (b)? If not, explain the effect of increased energy on the actual path as shown in the simulation. Pause or reset, select “40” for both protons and neutrons, “min” for energy, show traces, and fire away. Does this match your prediction from (c)? If not, explain why the actual path would be that shown in the simulation. Repeat this with larger numbers of protons and neutrons. What generalization can you make regarding the type of atom and effect on the path of α particles? Be clear and specific.
Check Answer: [13]
5.4 Defining the Nuclear Atom
- Describe the structure of the nuclear atom as proposed by Rutherford.
Check Answer: [14] - Write the ion symbol for each of the following ions and state whether it is an anion or a cation.
- the ion with atomic number 55, 54 electrons, and mass number 133
- the ion with 54 electrons, 53 protons
- the ion with atomic number 15 and a 3− charge
- the ion with 24 electrons, and a 3+ charge (Hint - the neutral atom would have lost electrons to form this positive ion).
Check Answer: [15]
- Write the ion symbol for each of the following ions:
- the ion with a 3+ charge, 31 protons
- the ion with 36 electrons, 35 protons
- the ion with 86 electrons, and a 4+ charge
- the ion with a 2+ charge, atomic number 38
Check Answer: [16]
- Open the Build an Atom simulation - Choose the ATOM simulation. Expand the net charge and mass number options by clicking on the + icon.
- Drag protons, neutrons, and electrons onto the atom template to make a neutral atom of Lithium that gives a mass number of 6. Write the element symbol for a neutral lithium atom.
- Now remove one electron to make an ion. What is the ion's net charge? Give the symbol for the ion you have created.
Check Answer: [17]
- Open the Build an Atom simulation - Choose the ATOM simulation. Expand the net charge and mass number options by clicking on the + icon.
- Drag protons, neutrons, and electrons onto the atom template to make a neutral atom of Nitrogen that gives a mass number of 14. How many protons, neutrons, and electrons are there in this nitrogen atom?
- Write the element symbol for a neutral Nitrogen atom.
- Now add three electrons to make an ion. How many TOTAL electrons are now in this nitrogen atom? What is the ion's net charge? Give the symbol for the ion you have created.
Check Answer: [18]
5.5 Isotopes of Elements
- In what way are isotopes of a given element always different? In what way(s) are they always the same? Check Answer: [19]
- Write the isotope symbol for each of the following neutral elements:
- atomic number 55, mass number 133
- 53 protons, 74 neutrons
- 15 electrons, mass number 31
- atomic number 27, 30 neutrons
Check Answer: [20]
- Write the isotope symbol for each of the following neutral elements:
- 31 electrons, and a mass number of 71
- 35 protons, and 45 neutrons
- atomic number 90, 142 neutrons
- 38 protons, and mass number 87
Check Answer: [21]
- Open the Build an Atom simulation and click on the Atom icon.
- Pick any one of the first 10 elements that you would like to build and state its symbol.
- Drag protons, neutrons, and electrons onto the atom template to make an atom of your element. State the numbers of protons, neutrons, and electrons in your atom, as well as the net charge and mass number.
- Click on “Net Charge” and “Mass Number,” check your answers to (b), and correct, if needed.
- Predict whether your atom will be stable or unstable. State your reasoning.
- Check the “Stable/Unstable” box. Was your answer to (d) correct? If not, first predict what you can do to make a stable atom of your element, and then do it and see if it works. Explain your reasoning.
Check Answer: [22]
- Open the Build an Atom simulation
- Drag protons, neutrons, and electrons onto the atom template to make a neutral atom of Oxygen-16 and give the isotope symbol for this atom.
- Now add two more electrons to make an ion and give the symbol for the ion you have created.
Check Answer: [23]
- Open the Build an Atom simulation
- Drag protons, neutrons, and electrons onto the atom template to make a neutral atom, stable atom of Fluorine. How many protons, neutrons, and electrons does this stable isotope have? What is the mass number of this stable isotope? Give the isotope symbol for this atom.
Check Answer: [24]
- Drag protons, neutrons, and electrons onto the atom template to make a neutral atom, stable atom of Fluorine. How many protons, neutrons, and electrons does this stable isotope have? What is the mass number of this stable isotope? Give the isotope symbol for this atom.
- Determine the number of protons, neutrons, and electrons in the following isotopes that are used in medical diagnoses and name each element. Then, give the isotope symbol including atomic number, mass number and ion charge:
- atomic number 9, mass number 18, charge of 1−
- atomic number 43, mass number 99, charge of 7+
- atomic number 53, atomic mass number 131, charge of 1−
- atomic number 81, atomic mass number 201, charge of 1+
- Name the elements in parts (a), (b), (c), and (d).
Check Answer: [25]
- The following are properties of isotopes of two elements that are essential in our diet. Determine the number of protons, neutrons and electrons in each and name the isotope.
- atomic number 26, mass number 58, charge of 2+
- atomic number 53, mass number 127, charge of 1−
Check Answer: [26]
- Give the number of protons, electrons, and neutrons in neutral atoms of each of the following isotopes. Name the element:
- [latex]_5^{10}\text{B}[/latex]
- [latex]_{80}^{199}\text{Hg}[/latex]
- [latex]_{29}^{63}\text{Cu}[/latex]
- [latex]_6^{13}\text{C}[/latex]
- [latex]_{34}^{77}\text{Se}[/latex]
Check Answer: [27]
- Give the number of protons, electrons, and neutrons in neutral atoms of each of the following isotopes:
- [latex]_3^7\text{Li}[/latex]
- [latex]_{52}^{125}\text{Te}[/latex]
- [latex]_{47}^{109}\text{Ag}[/latex]
- [latex]_{7}^{15}\text{N}[/latex]
- [latex]_{15}^{31}\text{P}[/latex]
Check Answer: [28]
- Click on the Isotopes and Atomic Mass Simulation and select the “Mixtures” simulation option. Then under the isotope mixture option choose "My Mix". Hide the “Percent Composition” and “Average Atomic Mass” boxes, and then select the element boron (B).
- Write the symbols of the isotopes of boron that are provided (naturally occurring) in significant amounts.
- Predict the relative amounts (percentages) of these boron isotopes found in nature. % of isotope 1 vs % of isotope 2 of boron. Explain the reasoning behind your choice. (hint - reference your periodic table for an elements atomic mass).
- Add isotopes to the black box to make a mixture that matches your prediction in (b). You may drag isotopes from their bins or click on “More” and then move the sliders to the appropriate amounts.
- Reveal the “Percent Composition” and “Average Atomic Mass” boxes. How well does your mixture match with your prediction? If necessary, adjust the isotope amounts to match your prediction.
- Select “Nature’s” mix of isotopes and compare it to your prediction. How well does your prediction compare with the naturally occurring mixture? Explain. If necessary, adjust your amounts to make them match “Nature’s” amounts as closely as possible.
Check Answer: [29]
- Repeat Chemistry End of Chapter Exercise 11 (a-e) using the PhET isotopes and atomic mass simulation and choose the element Silicon, which has three naturally occurring isotopes. Check Answer: [30]
5.6 Atomic Mass
- An element has the following natural abundances and isotopic masses: 90.92% abundance with 19.99 amu, 0.26% abundance with 20.99 amu, and 8.82% abundance with 21.99 amu. Calculate the average atomic mass of this element. Write the name and element symbol for this element.
Check Answer: [31] - Average atomic masses listed by IUPAC are based on a study of experimental results. Bromine has two isotopes 79Br and 81Br, whose masses (78.9183 and 80.9163 amu) and abundances (50.69% and 49.31%) were determined in earlier experiments. Calculate the average atomic mass of bromine based on these experiments.
Check Answer: [32] - Variations in average atomic mass may be observed for elements obtained from different sources. Lithium provides an example of this. The isotopic composition of lithium from naturally occurring minerals is 7.5% 6Li and 92.5% 7Li, which have masses of 6.01512 amu and 7.01600 amu, respectively. A commercial source of lithium, recycled from a military source, was 3.75% 6Li (and the rest 7Li). Calculate the average atomic mass values for each of these two sources.
Check Answer: [33] - The average atomic masses of some elements may vary, depending upon the sources of their ores. Naturally occurring boron consists of two isotopes with accurately known masses (10B, 10.0129 amu and 11B, 11.0931 amu). The actual atomic mass of boron can vary from 10.807 to 10.819, depending on whether the mineral source is from Turkey or the United States. Calculate the percent abundances leading to the two values of the average atomic masses of boron from these two countries.
Check Answer: [34] - The 18O:16O abundance ratio in some meteorites is greater than that used to calculate the average atomic mass of oxygen on earth. Is the average mass of an oxygen atom in these meteorites greater than, less than, or equal to that of a terrestrial oxygen atom?
Check Answer: [35]
Attribution & References
Except where otherwise noted, this page is adapted by Jackie MacDonald from “2. 1 Early ideas in atomic theory”, “2.2 Evolution of Atomic Theory” and “2.3 Atomic Structure and Symbolism” In Chemistry 2e (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson is licensed under CC BY 4.0. Access for free at Chemistry 2e (Open Stax). / Additional questions created and added by Jackie MacDonald.
- The starting materials consist of one green, larger sphere and two blue, smaller spheres. The products consist of two green spheres and two blue spheres. This violates Dalton’s theory that that atoms are not created during a chemical change, but are merely redistributed. ↵
- Atoms are neither created nor destroyed during a chemical change ↵
- This statement violates Dalton’s fourth postulate: In a given compound, the numbers of atoms of each type (and thus also the percentage) always have the same ratio. ↵
- X+Z have the same ratios of C and H and are therefore similar compounds, and is an example of the Law of Definite Proportions; however, they may or may not be the same compound. Samples from two unknown compounds that have the same mass ratio are not necessarily the same substance. Example: formaldehyde (CH2O) and glyceraldehyde (C3H6O3) have the same mass ratios but are not the same compound. X+Y and Y+Z have differing ratios of C and H and are therefore different compounds, aligning with the Law of Multiple Proportions. View an explanation of the solution in the video "Problem 2.1.3" ↵
- (a) TRUE; (b) FALSE - Law of conservation of mass; (c) TRUE (d) FALSE - Invalid ↵
- (a) TRUE; (b) FALSE – attractive; (c)FALSE – attraction; (d) FALSE - opposite ↵
- The force of attraction increases as the distance decreases between two oppositely charged objects (as the objects near one another the force of attraction is greater / gets stronger). ↵
- The force of attraction decreases as the distance increases between two oppositely charged objects (as the objects get further from one another the attractive force between the two objects lessens / gets weaker) ↵
- Induction is a method used to charge an object without actually touching the object to any other charged object. ↵
- Protons reside in an atom’s nucleus; whereas electrons reside outside the nucleus in the space around the nucleus. Electrons can freely move around nucleus of the atom. Electrons are a type of subatomic particle with a negative charge; whereas protons are a type of subatomic particle with a positive charge. Mass of the proton is found to be 1.673 x 10-24 g, which is 1,836 times the mass of an electron. ↵
- Both protons and neutrons are subatomic particles that reside in an atom’s nucleus. Both have approximately the same mass. Protons are positively charged, whereas neutrons are uncharged. ↵
- See written answers below. For a video demonstration of this answer view "2.8 | Predict and test the behaviour of α particles fired at a “plum pudding” model atom. (a) Predict" 3(a) This is a prediction; your prediction may be different than shown here. Most of the α alpha particles should repel the atoms in the structure and would not directly go through since the positive charge in the plum pudding model was assumed to be spread out throughout the entire volume of the atom. They would be redirected and deflected in many areas.3(b) This is a prediction; your prediction may be different than shown here. α alpha particles of higher energy would be moving faster and reacting faster. They would still come in contact with the positive charges scattered throughout the atom and will be deflected faster in many directions due to higher energy state.3(c) Your answer may be YES or NO depending on the predictions you made. The predictions listed above did not match what happened in the simulation for question (a) and (b). In the simulation, the majority of the α alpha particles passed straight through the plum pudding model. This is because the positive charge in the plum pudding model was assumed to be spread out throughout the entire volume of the atom and so were the oppositely charged electrons. Therefore, the electric field from the positively charged "pudding" would be too weak to significantly affect the path of the relatively massive particles moving at slower (lower energy) or faster speeds (higher energy). ↵
- See written answers below. For a video demonstration of this answer view the video "2.9 | Predict and test the behaviour of α particles fired at a Rutherford atom model." (a) The Rutherford atom has a small, positively charged nucleus, so most α particles will pass through empty space far from the nucleus and be undeflected. Those α particles that pass near the nucleus will be deflected from their paths due to positive-positive repulsion. The more directly toward the nucleus the α particles are headed, the larger the deflection angle will be.4(b) Higher-energy α particles that pass near the nucleus will still undergo deflection, but the faster they travel, the less the expected angle of deflection.4(c) If the nucleus is smaller, the positive charge is smaller and the expected deflections are smaller—both in terms of how closely the α particles pass by the nucleus undeflected and the angle of deflection. If the nucleus is larger, the positive charge is larger and the expected deflections are larger—more α particles will be deflected, and the deflection angles will be larger.4(d) The paths followed by the α particles match the predictions from (a), (b), and (c). ↵
- The majority of the atom's structure is made up of empty space, with a centrally located, very concentrated nucleus. The three major subatomic particles are protons, neutrons, and electrons. The nucleus contains positively charged protons and neutrally charged neutrons. Combined, these account for most of the mass in a given atom. The negative electrons, which contribute very little to the overall mass of the atom, are in orbit around the nucleus within the empty space. ↵
- (a) Cs+ - cation; (b) I- - anion; (c) P3- - anion; (d) Co3+ - cation ↵
- (a) Ga3+ (b) Br- (c) Th4+ (d) Sr2+ ↵
- (a) Symbol for neutral lithium atom (3 protons, 3 neutrons and 3 electrons) is Li; (b) By removing one electron lithium atom now has only 2 electrons but still 3 protons. The ion's net charge is +1. The symbol for the lithium ion is Li+. ↵
- (a) There are 7 protons, 7 neutrons, and 7 electrons in the nitrogen atom; (b) N symbol for neutral atom is N; (c) TOTAL electrons = 7+3 = 10 electrons in nitrogen ion. Its net ionic charge is 3- or -3. Ion symbol is N3-. ↵
- Isotopes of a given element always have different masses due to different numbers of neutrons. They always have the same number of protons, which determines the identity of the element. ↵
- (a) [latex]_{55}^{133}\text{Cs}[/latex] (b) [latex]_{53}^{127}\text{I}[/latex] (c) [latex]_{15}^{31}\text{P}[/latex] (d) [latex]_{27}^{57}\text{Co}[/latex] ↵
- (a) [latex]_{31}^{71}\text{Ga}[/latex] (b) [latex]_{35}^{80}\text{Br}[/latex] (c) [latex]_{90}^{232}\text{Th}[/latex] (d) [latex]_{38}^{87}\text{Sr}[/latex] ↵
- This is just one example of an answer the first ten elements - the element Nitrogen was chosen. You may have chosen a different atom and therefore would have different answers. (a) Nitrogen symbol is N; (b) We built an atom with 7 protons, 7 electrons and 7 neutrons; the net charge is 0; mass number is 14 (number of protons + number of neutrons); (c) our answers check correctly with the simulation. (c) We predict the nitrogen atom we build with be stable. The number of protons and neutrons are a similar number and are likely to be a stable isotope that occurs naturally. (d) when stable/stable is checked off, our element is STABLE; if it was unstable, you would add or take away neutrons until the simulation shows a stable isotope. Nitrogen-15 (has 8 neutrons) is also a stable isotope of nitrogen. However nitrogen atoms with 6 or less or 9 or more neutrons are unstable. As a result, they are not abundant in nature. ↵
- (a) Oxygen-16 isotope symbol is [latex]_{8}^{16}\text{O}[/latex] (b) O2- ↵
- A neutral, stable atom of fluorine will have 9 protons, 9 electrons, and 10 neutrons. Mass number is 19 (number of protons + number of neutrons); Isotope symbol for Fluorine-19 is [latex]_{9}^{19}\text{F}[/latex] ↵
- (a) 9 protons, 9 neutrons, 10 electrons in fluorine; [latex]_{9}^{18}\text{F}[/latex]- (b) 43 protons, 56 neutrons, 36 electrons in Technetium; [latex]_{43}^{99}\text{Tc}[/latex]7+ (c) 53 protons, 78 neutrons, 54 electrons in Iodine [latex]_{53}^{131}\text{I}[/latex]- (d) 81 protons, 120 neutrons, 80 electrons in Thallium; [latex]_{81}^{201}\text{Tl}[/latex]+ ↵
- (a) 26 protons, 32 neutrons, 24 electrons; Iron-58 (b) 53 protons, 74 neutrons, 54 electrons; Iodine-127 ↵
- (a) 5 protons, 5 electrons, 5 neutrons; Boron (b) 80 protons, 80 electrons, 119 neutrons; Mercury (c) 29 protons, 29 electrons, 34 neutrons; Copper (d) 6 protons, 6 electrons, 7 neutrons; Carbon (e) 34 protons, 34 electrons, 43 neutrons; Selenium ↵
- (a) 3 protons, 3 electrons, 4 neutrons; Lithium (b) 52 protons, 52 electrons, 73 neutrons; Tellurium (c) 47 protons, 47 electrons, 62 neutrons; Silver (d) 7 protons, 7 electrons, 8 neutrons; Nitrogen (e) 15 protons, 15 electrons, 16 neutrons; Phosphorus ↵
- (a) There are two isotopes, boron-10 and boron-11. (b) There is no way to be sure to accurately predict the abundances to give you an answer of 10.81 amu average atomic mass for boron, as shown on periodic table. However, we can see that the average of 10.81 amu is closest to 11 amu, so the natural abundances will contain more boron-11 isotopes than boron-10 isotopes. Let us guess that the abundances are 15% B-10, 85% B-11. The average mass would be (.15x10 + .85x11) 10.85 amu (you may have predicted different percentages - it is just a prediction). (e) Checking the nature’s mix of isotopes for boron shows that the abundances are 19.9% B-10 and 80.1% for B-11, so our guessed amounts only had to be slightly adjusted. ↵
- Let us use neon as an example. Since there are three isotopes (Ne-20, Ne21, Ne-23), there is no way to be sure to accurately predict the abundances to make the total of 20.18 amu average atomic mass. Let us guess that the abundances are 9% Ne-22, 91% Ne-20, and only a trace of Ne-21. The average mass would be 20.18 amu. Checking the nature’s mix of isotopes shows that the abundances are 90.48% Ne-20, 9.25% Ne-22, and 0.27% Ne-21, so our guessed amounts have to be slightly adjusted. ↵
- Average atomic mass of element = (0.9092 x 19.99) + (0.0026x20.99) + (0.0882 x 21.99) = 20.169 amu. This average atomic mass is similar to Neon (Ne). ↵
- Average atomic mass of Bromine = (0.5069 x 78.9183) + (0.4931 x 80.9163 ) = 79.904 amu ↵
- Average atomic mass of naturally occurring lithium = (0.075x 6.01512 ) + (0.925 x 7.01600 ) = 6.941 amu; Average atomic mass of commercial sourced lithium = (0.0375x 6.01512 ) + (0.9625 x 7.01600 ) = 6.978 amu ↵
- Turkey source: Equation used to solve is 10.807 = (x)10.0129x + (1-x)(11.0931) ANSWER: 26.49% of B-10 and 73.51% of B-11. US Source: 10.819 = (x)10.0129x + (1-x)(11.0931) ANSWER: 25.37 of B-10 and 74.63% of B-11. ↵
- The average mass of an oxygen atom in these meteorites will be great than an oxygen atom from earth since the meteorites contain more O-18 and less O-16 isotopes. Whereas on earth, the majority of the oxygen isotopes on earth are O-16. For a video demonstration of this answer view the video "2.26 | The O-18:O-16 abundance ratio" ↵

