5.6 Atomic Mass
Learning Objectives
By the end of this section, you will be able to:
- Define the atomic mass unit and average atomic mass
- Calculate average atomic mass and isotopic abundance
The development of modern atomic theory revealed much about the inner structure of atoms. It was learned that an atom contains a very small nucleus composed of positively charged protons and uncharged neutrons, surrounded by a much larger volume of space containing negatively charged electrons. The nucleus contains the majority of an atom’s mass because protons and neutrons are much heavier than electrons, whereas electrons occupy almost all of an atom’s volume. The diameter of an atom is on the order of 10−10 m, whereas the diameter of the nucleus is roughly 10−15 m—about 100,000 times smaller. For a perspective about their relative sizes, consider this: If the nucleus were the size of a blueberry, the atom would be about the size of a football stadium (Figure 5.6a).

Watch Just How Small is an Atom? (5:27 min)
Video Source: TED-Ed. (2012, April 16). Just how small is an atom? [Video]. YouTube.
Atoms—and the protons, neutrons, and electrons that compose them—are extremely small. For example, a carbon atom weighs less than 2 × 10−23 g, and an electron has a charge of less than 2 × 10−19 C (coulomb). When describing the properties of tiny objects such as atoms, we use appropriately small units of measure, such as the atomic mass unit (amu) and the fundamental unit of charge (e). The amu was originally defined based on hydrogen, the lightest element, then later in terms of oxygen. Since 1961, it has been defined with regard to the most abundant isotope of carbon, atoms of which are assigned masses of exactly 12 amu. (This isotope is known as “carbon-12”). Thus, one amu is exactly [latex]\frac{1}{12}[/latex] of the mass of one carbon-12 atom: 1 amu = 1.6605 × 10−24 g. (The Dalton (Da) and the unified atomic mass unit (u) are alternative units that are equivalent to the amu.) The fundamental unit of charge (also called the elementary charge) equals the magnitude of the charge of an electron (e) with e = 1.602 × 10−19 C.
A proton has a mass of 1.0073 amu and a charge of 1+. A neutron is a slightly heavier particle with a mass 1.0087 amu and a charge of zero; as its name suggests, it is neutral. The electron has a charge of 1− and is a much lighter particle with a mass of about 0.00055 amu (it would take about 1800 electrons to equal the mass of one proton). The properties of these fundamental particles are summarized in Table 5.6a. (An observant student might notice that the sum of an atom’s subatomic particles does not equal the atom’s actual mass. The total mass of six protons, six neutrons, and six electrons is 12.0993 amu, slightly larger than 12.00 amu. This “missing” mass is known as the mass defect, which you can learn about it if you study nuclear chemistry.)
| Name | Location | Charge (C) | Unit Charge | Mass (amu) | Mass (g) |
|---|---|---|---|---|---|
| electron | outside nucleus | −1.602 × 10−19 | 1− | 0.00055 | 0.00091 × 10−24 |
| proton | nucleus | 1.602 × 10−19 | 1+ | 1.00727 | 1.67262 × 10−24 |
| neutron | nucleus | 0 | 0 | 1.00866 | 1.67493 × 10−24 |
Atomic Mass
Because each proton and each neutron contribute approximately one amu to the mass of an atom, and each electron contributes far less, the atomic mass of a single atom is approximately equal to its mass number (a whole number). However, the average masses of atoms of most elements are not whole numbers because most elements exist naturally as mixtures of two or more isotopes, each with their own slightly different masses due to the different number of neutrons they contain.
The mass of an element shown in a periodic table or listed in a table of atomic masses is a weighted, average mass of all the isotopes present in a naturally occurring sample of that element. This is equal to the sum of each individual isotope’s mass multiplied by its fractional abundance.
For example, the element boron is composed of two isotopes: About 19.9% of all boron atoms are 10B with a mass of 10.0129 amu, and the remaining 80.1% are 11B with a mass of 11.0093 amu. The average atomic mass for boron is calculated to be:
It is important to understand that no single boron atom weighs exactly 10.8 amu; 10.8 amu is the average mass of all boron atoms, and individual boron atoms weigh either approximately 10 amu or 11 amu.
Exercise 5.6a
Calculating Average Atomic Mass
5.6a - Calculating Average Atomic Mass (Text version)
A meteorite found in central Indiana contains traces of the noble gas neon picked up from the solar wind during the meteorite’s trip through the solar system. Analysis of a sample of the gas showed that it consisted of 91.84% 20Ne (mass 19.9924 amu), 0.47% 21Ne (mass 20.9940 amu), and 7.69% 22Ne (mass 21.9914 amu). What is the average mass of the neon in the solar wind?
STEP 1 - List known quantities and identify what you are asked to find
Known information: Isotopes of neon - 91.84% 20Ne (mass 19.9924 amu), 0.47% 21Ne (mass 20.9940 amu), and 7.69% 22Ne (mass 21.9914 amu).
What am I asked to Find: average mass of the neon in the solar wind
Step 2 - Determine how you will solve the problem
Use the following formula to solve problem:
[latex]\displaystyle{} \text{average mass} = \sum_{i} (\text{fractional abundance} \times \text{isotopic mass})_{i}[/latex]
Step 3 - Solve the Problem
[latex]\begin{array}{r @{{}={}} l} \text{average mass} & = (0.9184 \times 19.9924 \;\text{amu}) + (0.0047 \times 20.9940 \;\text{amu})+(0.0769 \times 21.9914 \;\text{amu}) \\[1em] & = (18.36+0.099+1.69) \;\text{amu} \\[1em] & = 20.15 \;\text{amu} \end{array}[/latex]
The average mass of a neon atom in the solar wind is 20.15 amu. (The average mass of a terrestrial neon atom is 20.1796 amu. This result demonstrates that we may find slight differences in the natural abundance of isotopes, depending on their origin.)
Step 4 - Does the answer make sense?
-
- Yes it does. It is similar to the average atomic mass of Ne on the periodic table. The average atomic mass is in between the given amu values for Ne-20, Ne-21 and Ne-22, and the average amu value calculated is closest to the most abundant isotope, Ne-20.
Source: "Exercise 5.6a" by Jackie MacDonald, licensed under CC BY-SA 4.0.
If you want to review additional examples of how to calculate average atomic mass, Watch How to Calculate Atomic Mass Practice Problems (6 mins 10s)
Video Source: Tyler DeWitt. (2012, October 5). How to calculate Atomic Mass practice problems [Video]. YouTube.
Exercise 5.6b
Calculating Average Atomic Mass
A sample of magnesium is found to contain 78.70% of 24Mg atoms (mass 23.98 amu), 10.13% of 25Mg atoms (mass 24.99 amu), and 11.17% of 26Mg atoms (mass 25.98 amu). Calculate the average mass of a Mg atom.
Check Your Answer[1]
We can also do variations of this type of calculation where you calculate the percent abundance of each isotope, as shown in the next example:
Example 5.6b
Calculating Percent Abundance
Naturally occurring chlorine consists of 35Cl (mass 34.96885 amu) and 37Cl (mass 36.96590 amu), with an average mass of 35.453 amu. What is the percent composition of Cl in terms of these two isotopes?
Solution
Step 1 - List known quantities and identify what you are asked to find
Known information: 35Cl (mass 34.96885 amu) and 37Cl (mass 36.96590 amu), with copper having an average atomic mass of 35.453 amu
What am I asked to Find: What is the percent composition of Cl in terms of these two isotopes
Step 2 - Determine how you will solve the problem
Use the following formula to solve problem:
The average mass of chlorine is the fraction that is 35Cl times the mass of 35Cl plus the fraction that is 37Cl times the mass of 37Cl.
[latex]\text{average mass} = (\text{fraction of} \ ^{35}\text{Cl} \ \times \ \text{mass of} \ ^{35}\text{Cl}) + (\text{fraction of} \ ^{37}\text{Cl} \ \times \ \text{mass of} \ ^{37}\text{Cl})[/latex]
If we let x represent the fraction that is 35Cl, then the fraction that is 37Cl is represented by 1.00 − x.
(The fraction that is 35Cl + the fraction that is 37Cl must add up to 1, so the fraction of 37Cl must equal 1.00 − the fraction of 35Cl.)
Step 3 - Solve the Problem
Substituting this into the average mass equation, we have:
So solving yields: x = 0.7576, which means that 1.00 − 0.7576 = 0.2424. Therefore, chlorine consists of 75.76% 35Cl and 24.24% 37Cl.
Step 4 - Does the answer make sense?
Yes it does. The highest % abundance found is 35Cl ; which has a mass of 34.96885 amu is closest to the average mass of Cu, which is 35.453 amu.
If you want to watch the full work through of Example 5.6b - Calculation of Percent Abundance, Watch How to Calculate Isotope Abundance (11 mins 48s)
Video Source: DeWitt, T. (2012, October 4). Atomic mass: How to calculate isotope abundance [Video]. YouTube.
Exercise 5.6c
Calculation of Percent Abundance
Naturally occurring copper consists of 63Cu (mass 62.9296 amu) and 65Cu (mass 64.9278 amu), with an average mass of 63.546 amu. What is the percent composition of Cu in terms of these two isotopes?
Check Your Answer[2]
Try the interactive learning activity suggested below to reinforce your learning about isotope ratios and atomic mass. Try make mixtures of the main isotopes of the first 18 elements, gain experience with average atomic mass, and check naturally occurring isotope ratios using the Isotopes and Atomic Mass simulation.
Exercise 5.6d
Practice using the following PhET simulation: Isotopes and Atomic Mass
Determining Natural Abundances of Isotopes using Mass Spectrometry.
The occurrence and natural abundances of isotopes can be experimentally determined using an instrument called a mass spectrometer. Mass spectrometry (MS) is widely used in chemistry, forensics, medicine, environmental science, and many other fields to analyze and help identify the substances in a sample of material. In a typical mass spectrometer (Figure 5.6b), the sample is vaporized and exposed to a high-energy electron beam that causes the sample’s atoms (or molecules) to become electrically charged, typically by losing one or more electrons. These cations then pass through a (variable) electric or magnetic field that deflects each cation’s path to an extent that depends on both its mass and charge (similar to how the path of a large steel ball bearing rolling past a magnet is deflected to a lesser extent that that of a small steel BB). The ions are detected, and a plot of the relative number of ions generated versus their mass-to-charge ratios (a mass spectrum) is made. The height of each vertical feature or peak in a mass spectrum is proportional to the fraction of cations with the specified mass-to-charge ratio. Since its initial use during the development of modern atomic theory, MS has evolved to become a powerful tool for chemical analysis in a wide range of applications.
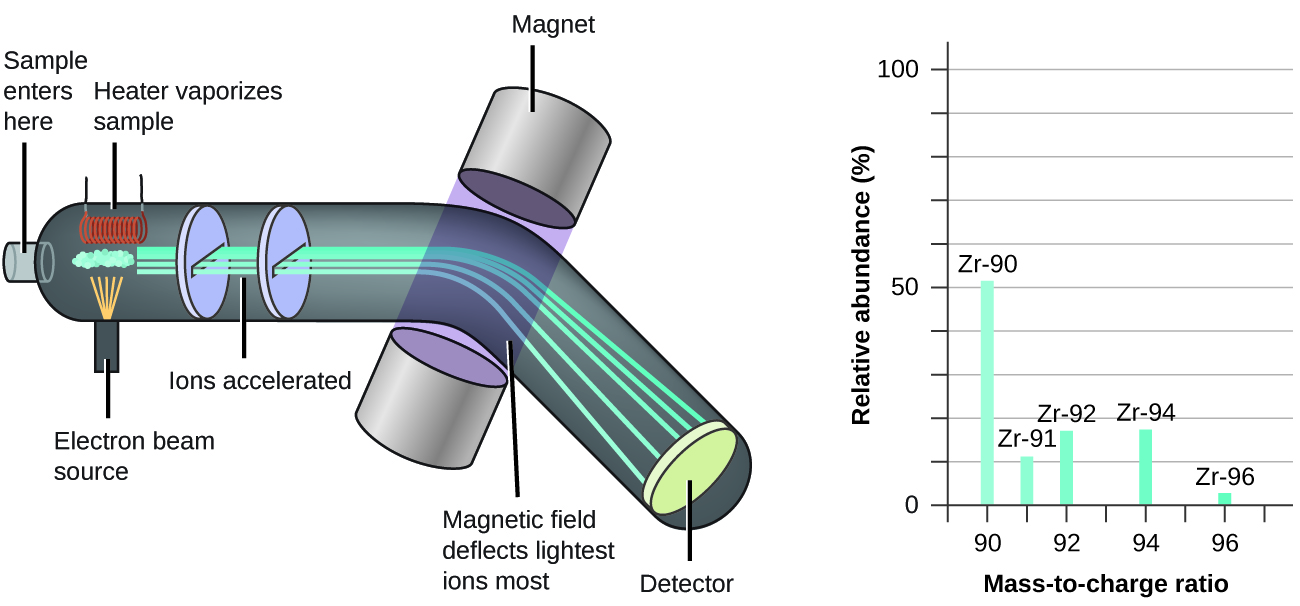
Watch Mass Spectometry (8:19 min)
Video Source: Bozeman Science. (2013, August 8). Mass spectrometry [Video]. YouTube.
Watch Mass Spectrometry MS (7:58 min)
Video Source: Royal Society of Chemistry. (2008, September 27). Mass Spectrometry MS [Video]. YouTube.
Key Equations
- [latex]\displaystyle{} \text{average mass} = \sum_{i} (\text{fractional abundance} \times \text{isotopic mass})_i[/latex]
Attribution & References
- Except where otherwise noted, this page is adapted from “2.3 - Atomic Structure and Symbolism ” In Chemistry 2e (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson is licensed under CC BY 4.0. Access for free at Chemistry 2e (Open Stax)
- Step 1 - List known quantities and identify what you are asked to find
Known information: 78.70% of 24Mg atoms (mass 23.98 amu), 10.13% of 25Mg atoms (mass 24.99 amu), and 11.17% of 26Mg atoms (mass 25.98 amu)
What am I asked to Find: average mass of a Mg atom
Step 2 - Determine how you will solve the problemUse the following formula to solve problem:
[latex]\displaystyle{} \text{average mass} = \sum_{i} (\text{fractional abundance} \times \text{isotopic mass})_{i}[/latex]
Step 3 - Solve the Problem[latex]\begin{array}{r @{{}={}} l} \text{average mass} & = (0.7870\times 23.98\;\text{amu}) + (0.1013\times 24.99\;\text{amu})+(0.1117\times 25.98\;\text{amu}) \\[1em] & = (18.872+2.531+2.902) \;\text{amu} \\[1em] & = 24.305\;\text{amu} \end{array}[/latex]
Step 4 - Does the answer make sense?- Yes it does. It is similar to the average atomic mass of Mg on the periodic table. The average atomic mass is in between the given amu values for Ne-20, Ne-21 and Ne-22, and the average amu value calculated is a bit more than the most abundant isotope, Mg-24.
- Step 1 - List known quantities and identify what you are asked to find
Known information: 63Cu (mass 62.9296 amu) and 65Cu (mass 64.9278 amu), with an average mass of 63.546 amu.
What am I asked to Find:What is the percent composition of Cu in terms of these two isotopes?
Step 2 - Determine how you will solve the problemUse the following formula to solve problem:
The average mass of Copper is the fraction that is 63Cu times the mass of 63Cu plus the fraction that is 65Cu times the mass of 65Cu
[latex]\text{average mass} = (\text{fraction of} \ ^{63}\text{Cu} \ \times \ \text{mass of} \ ^{63}\text{Cu}) + (\text{fraction of} \ ^{65}\text{Cu} \ \times \ \text{mass of} \ ^{65}\text{Cu})[/latex]If we let x represent the fraction that is 63Cu, then the fraction that is 65Cu is represented by 1.00 − x.
(The fraction that is 63Cu + the fraction that is 65Cu must add up to 1, so the fraction of 63Cu must equal 1.00 − the fraction of 65Cu.) Step 3 - Solve the Problem[latex]\begin{array}{r @{{}={}} l}63.546\;\text{amu} & = (x \times 62.9296\;\text{amu}) + [(1.00 - x) \times 64.9278\;\text{amu}] \\[1em] 63.546 & = 62.9296x+ 64.9278 - 64.9278x \\[1em] 1.9982x & = 1.3818\\[1em] x & = \frac{1.3818}{1.9982} = 0.6915\end{array}[/latex]So solving yields: x = 0.6915, which means that 1.00 − 0.6915= 0.3085. Therefore, chlorine consists of 69.15% of Cu-63 and and 30.85% Cu-65
Step 4 - Does the answer make sense?Yes it does. The highest % abundance found is Cu-63, which has a mass of 62.9296 amu is closest to the average mass of Cu, which is 63.546 amu.
↵
(also, unified atomic mass unit, u, or Dalton, Da) unit of mass equal to 1/12 the mass of a 12C atom
(also called the elementary charge) equals the magnitude of the charge of an electron (e) with e = 1.602 × 10−19 C
alternative unit equivalent to the atomic mass unit
alternative unit equivalent to the atomic mass unit
average mass of atoms of an element, expressed in amu

