14.1 Solutions: An introduction
Learning Objectives
- Describe the basic properties of solutions and how they form
- Predict whether a given mixture will yield a solution based on molecular properties of its components
- Explain why some solutions either produce or absorb heat when they form
An earlier chapter of this text introduced solutions, defined as homogeneous mixtures of two or more substances. Often, one component of a solution is present at a significantly greater concentration, in which case it is called the solvent. The other components of the solution present in relatively lesser concentrations are called solutes. Sugar is a covalent solid composed of sucrose molecules, C12H22O11. When this compound dissolves in water, its molecules become uniformly distributed among the molecules of water:
The subscript “aq” in the equation signifies that the sucrose molecules are solutes and are therefore individually dispersed throughout the aqueous solution (water is the solvent). Although sucrose molecules are heavier than water molecules, they remain dispersed throughout the solution; gravity does not cause them to “settle out” over time.
Potassium dichromate, K2Cr2O7, is an ionic compound composed of colourless potassium ions, K+, and orange dichromate ions, [latex]\text{Cr}_2\text{O}_7^{\;\;2-}[/latex]. When a small amount of solid potassium dichromate is added to water, the compound dissolves and dissociates to yield potassium ions and dichromate ions uniformly distributed throughout the mixture (Figure 14.1a), as indicated in this equation:
As for the mixture of sugar and water, this mixture is also an aqueous solution. Its solutes, potassium and dichromate ions, remain individually dispersed among the solvent (water) molecules.

Exercise 14.1a
Practice using the following PhET simulation: Sugar and Salt Solutions
Water is used so often as a solvent that the word solution has come to imply an aqueous solution to many people. However, almost any gas, liquid, or solid can act as a solvent. Many alloys are solid solutions of one metal dissolved in another; for example, US five-cent coins contain nickel dissolved in copper. Air is a gaseous solution, a homogeneous mixture of nitrogen, oxygen, and several other gases. Oxygen (a gas), alcohol (a liquid), and sugar (a solid) all dissolve in water (a liquid) to form liquid solutions. Table 14.1a gives examples of several different solutions and the phases of the solutes and solvents.
| Solution | Solute | Solvent |
|---|---|---|
| air | O2(g) | N2(g) |
| soft drinks[1] | CO2(g) | H2O(l) |
| hydrogen in palladium | H2(g) | Pd(s) |
| rubbing alcohol | H2O(l) | C3H8O(l) (2-propanol) |
| saltwater | NaCl(s) | H2O(l) |
| brass | Zn(s) | Cu(s) |
Solutions exhibit these defining traits:
- They are homogeneous; that is, after a solution is mixed, it has the same composition at all points throughout (its composition is uniform).
- The physical state of a solution—solid, liquid, or gas—is typically the same as that of the solvent, as demonstrated by the examples in Table 14.1a.
- The components of a solution are dispersed on a molecular scale; that is, they consist of a mixture of separated molecules, atoms, and/or ions.
- The dissolved solute in a solution will not settle out or separate from the solvent.
- The composition of a solution, or the concentrations of its components, can be varied continuously, within limits.
The Formation of Solutions
The formation of a solution is an example of a spontaneous process, a process that occurs under specified conditions without the requirement of energy from some external source. Sometimes we stir a mixture to speed up the dissolution process, but this is not necessary; a homogeneous solution would form if we waited long enough. The topic of spontaneity is critically important to the study of chemical thermodynamics and is treated more thoroughly in a later chapter of this text. For purposes of this chapter’s discussion, it will suffice to consider two criteria that favour, but do not guarantee, the spontaneous formation of a solution:
- a decrease in the internal energy of the system (an exothermic change, as discussed in the previous chapter on thermochemistry)
- an increase in the disorder in the system (which indicates an increase in the entropy of the system, as you will learn about in the later chapter on thermodynamics)
In the process of dissolution, an internal energy change often, but not always, occurs as heat is absorbed or evolved. An increase in disorder always results when a solution forms.
When the strengths of the intermolecular forces of attraction between solute and solvent species in a solution are no different than those present in the separated components, the solution is formed with no accompanying energy change. Such a solution is called an ideal solution. A mixture of ideal gases (or gases such as helium and argon, which closely approach ideal behaviour) is an example of an ideal solution, since the entities comprising these gases experience no significant intermolecular attractions.
When containers of helium and argon are connected, the gases spontaneously mix due to diffusion and form a solution (Figure 14.1b). The formation of this solution clearly involves an increase in disorder, since the helium and argon atoms occupy a volume twice as large as that which each occupied before mixing.
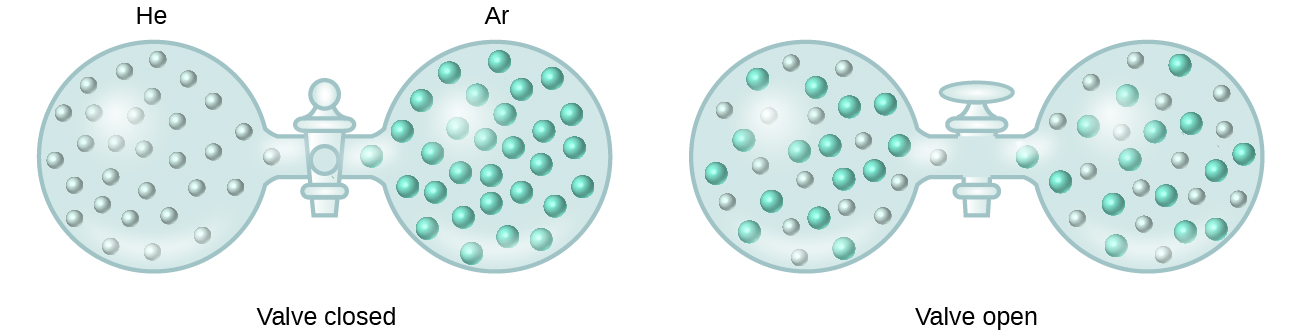
Ideal solutions may also form when structurally similar liquids are mixed. For example, mixtures of the alcohols methanol (CH3OH) and ethanol (C2H5OH) form ideal solutions, as do mixtures of the hydrocarbons pentane, C5H12, and hexane, C6H14. Placing methanol and ethanol, or pentane and hexane, in the bulbs shown in Figure 14.1b will result in the same diffusion and subsequent mixing of these liquids as is observed for the He and Ar gases (although at a much slower rate), yielding solutions with no significant change in energy. Unlike a mixture of gases, however, the components of these liquid-liquid solutions do, indeed, experience intermolecular attractive forces. But since the molecules of the two substances being mixed are structurally very similar, the intermolecular attractive forces between like and unlike molecules are essentially the same, and the dissolution process, therefore, does not entail any appreciable increase or decrease in energy. These examples illustrate how diffusion alone can provide the driving force required to cause the spontaneous formation of a solution. In some cases, however, the relative magnitudes of intermolecular forces of attraction between solute and solvent species may prevent dissolution.
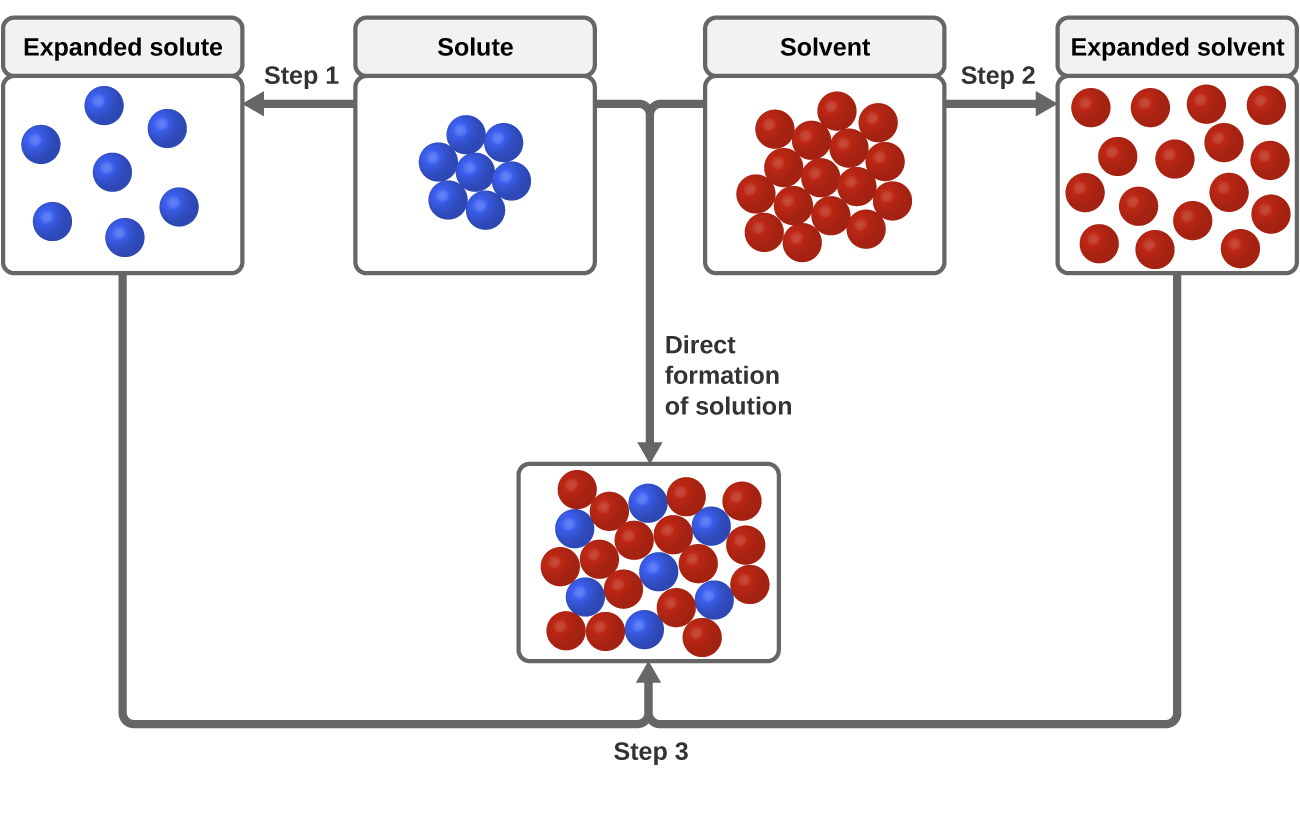
Three types of intermolecular attractive forces are relevant to the dissolution process: solute-solute, solvent-solvent, and solute-solvent. As illustrated in Figure 14.1c, the formation of a solution may be viewed as a stepwise process in which energy is consumed to overcome solute-solute and solvent-solvent attractions (endothermic processes) and released when solute-solvent attractions are established (an exothermic process referred to as solvation). The relative magnitudes of the energy changes associated with these stepwise processes determine whether the dissolution process overall will release or absorb energy. In some cases, solutions do not form because the energy required to separate solute and solvent species is so much greater than the energy released by solvation.
For example, cooking oils and water will not mix to any appreciable extent to yield solutions (Figure 14.1d). Hydrogen bonding is the dominant intermolecular attractive force present in liquid water; the nonpolar hydrocarbon molecules of cooking oils are not capable of hydrogen bonding, instead being held together by dispersion forces. Forming an oil-water solution would require overcoming the very strong hydrogen bonding in water, as well as the significantly strong dispersion forces between the relatively large oil molecules. And, since the polar water molecules and nonpolar oil molecules would not experience very strong intermolecular attraction, very little energy would be released by solvation.
On the other hand, a mixture of ethanol and water will mix in any proportion to yield a solution. In this case, both substances are capable of hydrogen bonding, and so the solvation process is sufficiently exothermic to compensate for the endothermic separations of solute and solvent molecules.
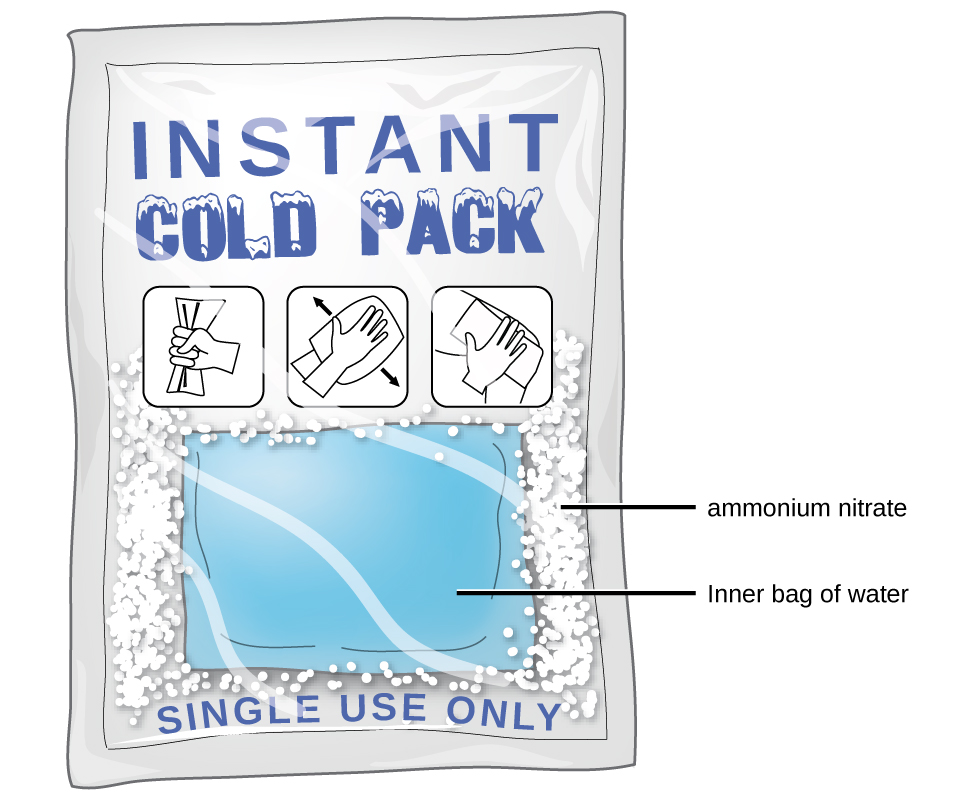
As noted at the beginning of this module, spontaneous solution formation is favoured, but not guaranteed, by exothermic dissolution processes. While many soluble compounds do, indeed, dissolve with the release of heat, some dissolve endothermically. Ammonium nitrate (NH4NO3) is one such example and is used to make instant cold packs for treating injuries like the one pictured in Figure 14.1e. A thin-walled plastic bag of water is sealed inside a larger bag with solid NH4NO3. When the smaller bag is broken, a solution of NH4NO3 forms, absorbing heat from the surroundings (the injured area to which the pack is applied) and providing a cold compress that decreases swelling. Endothermic dissolutions such as this one require a greater energy input to separate the solute species than is recovered when the solutes are solvated, but they are spontaneous nonetheless due to the increase in disorder that accompanies formation of the solution.
Watch Comparing Temperature Change from Heat of Solution (KCl vs NaOH) (2 mins)
Video source: dbthermo. (2012, April 24). Comparing Temperature Change from Heat of Solution (KCl vs NaOH) [Video]. YouTube. https://www.youtube.com/watch?v=o7adWQqvDUU
Indigenous Perspective: Maple Syrup

For thousands of years maple syrup has been gathered from maple trees in Canada and the northern United States, a practice that has sustained Indigenous communities while also helping to connect them to the land. This is a practice carried out by the Anishinaabeg, Haudenosaunee and other First Nations. The physical and chemical properties of this unique solution give it the ability to act as not only a sweetener, but also as an anesthetic and a desiccant to help cure meats. Aside from containing many nutrients and carbohydrates, maple syrup was also used as an essential trade item. To learn more about the chemistry of maple syrup and its importance in Indigenous culture, please visit:
Attribution & References
Except where otherwise noted, this page is adapted by Gregory A. Anderson from "11.1 The Dissolution Process" In General Chemistry 1 & 2 by Rice University, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson and is licensed under CC BY 4.0. Access for free at Chemistry (OpenStax) .
- If bubbles of gas are observed within the liquid, the mixture is not homogeneous and, thus, not a solution. ↵
Another name for a homogeneous mixture
solution component present in a concentration that is higher relative to other components
solution component present in a concentration less than that of the solvent
solid mixture of a metallic element and one or more additional elements
physical or chemical change that occurs without the addition of energy from an external source
solution that forms with no accompanying energy change
exothermic process in which intermolecular attractive forces between the solute and solvent in a solution are established

