15.4 Describing Reactions in Solutions by Writing Molecular, Complete Ionic, and Net Ionic Equations
Learning Objectives
By the end of this section, you will be able to:
- Write and balance chemical equations in molecular, complete ionic, and net ionic formats.
Ionic Compounds in Solution
We have learned that one important aspect about ionic compounds that differs from molecular compounds has to do with dissolving in a liquid, such as water. When molecular compounds, such as sugar, dissolve in water, the individual molecules drift apart from each other. When ionic compounds dissolve, the ions physically separate from each other. We can use a chemical equation to represent this process—for example, with NaCl:
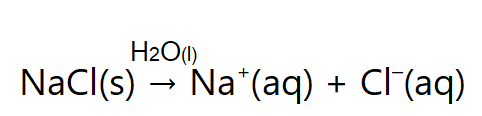
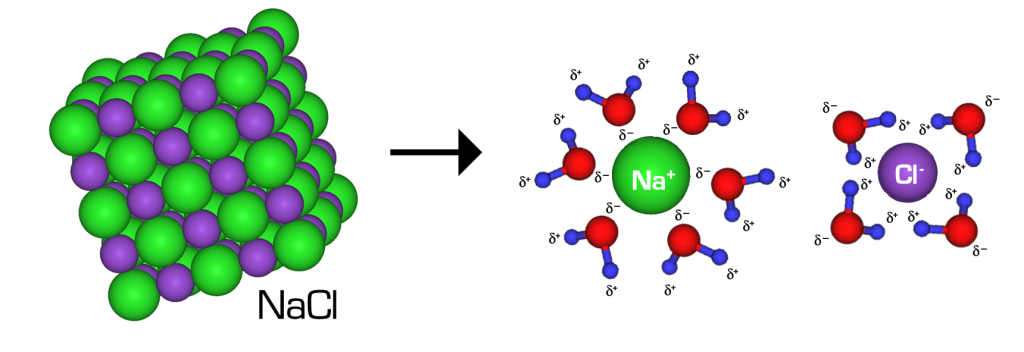
When NaCl dissolves in water, the ions separate and go their own way in solution; the ions are now written with their respective charges, and the (aq) phase label emphasizes that they are dissolved (Figure 15.4a and Figure 15.4b). When an ionic compound dissociates in water, water molecules surround each ion and separate it from the rest of the solid. Each ion goes its own way in solution.
All ionic compounds that dissolve behave this way. (This behaviour was first suggested by the Swedish chemist Svante August Arrhenius [1859–1927] as part of his PhD dissertation in 1884. Interestingly, his PhD examination team had a hard time believing that ionic compounds would behave like this, so they gave Arrhenius a barely passing grade. Later, this work was cited when Arrhenius was awarded the Nobel Prize in Chemistry.)
Keep in mind that when the ions separate, all of the ions separate. Thus, when CaCl2 dissolves, the one Ca2+ ion and the two Cl− ions separate from each other:
CaCl2(s) → Ca2+(aq) + Cl–(aq) + Cl–(aq)
which is simplified to
CaCl2(s) → Ca2+(aq) + 2Cl–(aq)
That is, the two chloride ions go off on their own. They do not remain as Cl2 (that would be elemental chlorine; these are chloride ions); they do not stick together to make Cl2− or Cl22−. They become dissociated ions in their own right. It is important to note that polyatomic ions retain their overall identity when they are dissolved. It is important to understand and write the chemical equation that represents the dissociation of ions, as it is a crucial concept when writing complete ionic chemical equations, and net ionic equations.
Example 15.4a
Write the chemical equation that represents the dissociation of each ionic compound.
- KBr
- Na2SO4
- (NH4)3PO4
Solution
- KBr(s) → K+(aq) + Br−(aq)
- Not only do the two sodium ions go their own way, but the polyatomic sulfate ion stays together as the sulfate ion. The dissolving equation is Na2SO4(s) → 2Na+(aq) + SO42−(aq)
- Not only do the three ammonium ions stay together, but the one phosphate ion stays together as well since they are both polyatomic ions. The dissolving equation is (NH4)3PO4(s) → 3NH4+(aq) + PO43−(aq)
Exercise 15.4a
Equations for Ionic Reactions
Given the abundance of water on earth, it stands to reason that a great many chemical reactions take place in aqueous media. When ions are involved in these reactions, the chemical equations may be written with various levels of detail appropriate to their intended use. To illustrate this, consider a reaction between ionic compounds taking place in an aqueous solution. When aqueous solutions of CaCl2 and AgNO3 are mixed, a precipitation reaction takes place producing aqueous Ca(NO3)2 and solid AgCl as shown in the following molecular equation:
CaCl2(aq) + 2AgNO3(aq) → Ca(NO3)2(aq) + 2AgCl(s)
This balanced equation, derived by means describe in previous sections, is called a molecular equation because it doesn’t explicitly represent the ionic species that are present in solution. When ionic compounds dissolve in water, they may dissociate into their constituent ions, which are subsequently dispersed homogenously throughout the resulting solution. Ionic compounds dissolved in water are, therefore, more realistically represented as dissociated ions, in this case:
CaCl2(aq) → Ca2+(aq) + 2Cl−(aq)
2AgNO3(aq) → 2Ag+(aq) + 2NO3−(aq)
Ca(NO3)2(aq) → Ca2+(aq) + 2NO3−(aq)
Unlike these three ionic compounds, AgCl does not dissolve in water to a significant extent, as signified by its physical state notation, s.
Explicitly representing all dissolved ions results in a complete ionic equation. In this particular case, the formulas for the dissolved ionic compounds are replaced by formulas for their dissociated ions:
Ca2+(aq) + 2Cl−(aq) + 2Ag+(aq) + 2NO3−(aq) → Ca2+(aq) + 2NO3−(aq) + 2AgCl(s)
NOTE: the order in which the products are written, does not matter. The solid may be written first or second. Examining this complete ionic equation shows that two chemical species are present in identical forms on both sides of the arrow, Ca2+(aq) and NO3−(aq). These spectator ions—ions whose presence is required to maintain charge neutrality—are neither chemically nor physically changed by the process, and so they may be eliminated from the equation to yield a more concise representation called a net ionic equation. The net ionic equation summarizes the chemical change that occurred in the chemical reaction. Below, the complete ionic equation is shown first. The spectator ions (Ca2+(aq) and 2NO3–(aq)) are bolded in blue. Then, the other reactants and products are included in the net ionic equation, which is shown second.
Complete Ionic Equation: Ca2+(aq) + 2Cl−(aq) + 2Ag+(aq) + 2NO3−(aq) → Ca2+(aq) + 2NO3−(aq)+ 2AgCl(s)
Net Ionic Equation: 2Cl−(aq) + 2Ag+(aq) → 2AgCl(s)
which can be simplified to the smallest possible integers as coefficients and written as
Cl−(aq) + Ag+(aq) → AgCl(s)
This net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver ions, regardless of the source of these ions. These molecular and complete ionic equations provide additional information, namely, the ionic compounds used as sources of Cl− and Ag+. The above reaction is a precipitation reaction.
Watch How to Write and Balance Net Ionic Equations (6min 18sec).
Video Source: Brezlyn, Wayne (2019, July 25). How to write and balance net ionic equations [Video]. YouTube.
Example 15.4b
The molecular equations for two chemical reactions are given below: Write the complete ionic equation for each chemical reaction.
- KBr(aq) + AgC2H3O2(aq) → KC2H3O2(aq) + AgBr(s)
- MgSO4(aq) + Ba(NO3)2(aq) → Mg(NO3)2(aq) + BaSO4(s)
Solution
For any ionic compound that is aqueous, we will write the compound as separated ions.
- The complete ionic equation is
K+(aq) + Br−(aq) + Ag+(aq) + C2H3O2−(aq) → K+(aq) + C2H3O2−(aq) + AgBr(s) - The complete ionic equation is
Mg2+(aq) + SO42−(aq) + Ba2+(aq) + 2NO3−(aq) → Mg2+(aq) + 2NO3−(aq) + BaSO4(s)
Exercise 15.4b
Write the complete ionic equation for the following chemical chemical reaction:
CaCl2(aq) + Pb(NO3)2(aq) → Ca(NO3)2(aq) + PbCl2(s)
Check Your Answer[3]
Example 15.4c
Write the net ionic equation for each chemical reaction below. Identify the spectator ions in each of these reactions.
- K+(aq) + Br−(aq) + Ag+(aq) + C2H3O2−(aq) → K+(aq) + C2H3O2−(aq) + AgBr(s)
- Mg2+(aq) + SO42−(aq) + Ba2+(aq) + 2 NO3−(aq) → Mg2+(aq) + 2 NO3−(aq) + BaSO4(s)
Solution
- In the first equation, the K+(aq) and C2H3O2−(aq) ions are spectator ions (bolded in blue), so they are cancelled.
K+(aq) + Br−(aq) + Ag+(aq) + C2H3O2−(aq) → K+(aq) + C2H3O2−(aq) + AgBr(s);
The net ionic equation is
Br−(aq) + Ag+(aq) → AgBr(s) - In the second equation, the Mg2+(aq) and NO3−(aq) ions are spectator ions (bolded in blue), so they are cancelled.
Mg2+(aq) + SO42−(aq) + Ba2+(aq) + 2NO3−(aq) → Mg2+(aq) + 2NO3−(aq) + BaSO4(s);
The net ionic equation is
SO42−(aq) + Ba2+(aq) → BaSO4(s)
Exercise 15.4c
Write the net ionic equation for the chemical reaction below. Identify the spectator ions in this reaction.
Ca2+(aq) + 2Cl−(aq) + Pb2+(aq) + 2NO3−(aq) → Ca2+(aq) + 2NO3−(aq) + PbCl2(s)
Check Your Answer[4]
Example 15.4d
When aqueous barium chloride is mixed with an aqueous solution of sodium sulfate, the mixture reacts to yield solid barium sulfate and aqueous sodium chloride. Write balanced molecular, complete ionic, and net ionic equations for this process. Name the spectator ions.
Solution
Begin by identifying formulas for the reactants and products and arranging them properly in chemical equation form:
BaCl2(aq) + Na2SO4(aq) → BaSO4(s) + NaCl(aq) (unbalanced equation)
Balance is achieved easily in this case by changing the coefficient for NaCl to 2, resulting in the molecular equation for this reaction:
BaCl2(aq) + Na2SO4(aq) → BaSO4(s) + 2NaCl(aq) (balanced equation)
The dissolved ionic compounds, BaCl2, Na2SO4, and NaCl, can be represented as dissociated ions to yield the complete ionic equation:
Ba2+(aq) + 2Cl−(aq) + 2Na+(aq) + SO42-(aq) → BaSO4(s) + 2Na+(aq) + 2Cl−(aq)
Finally, identify the spectator ion(s), in this case Na+(aq) and Cl– (aq), and remove them from each side of the equation to generate the net ionic equation:
Ba2+(aq) + SO42-(aq) → BaSO4(s)
Exercise 15.4d
When an aqueous solution of AgNO3 is added to an aqueous solution of CaCl2, insoluble AgCl precipitates. Write three equations (complete molecular equation, complete ionic equation, and net ionic equation) that describe this process.
Check Your Answer[5]
Net Ionic Equations of Gas Forming Reactions
Sometimes a gas will be involved as one of the reactants or products in a solution reaction. Net ionic equations can be written for these chemical reactions, as well. For example,
2HCl(aq) + Na2S(aq) → H2S(g) + 2NaCl(aq)
In this example, since hydrochloric acid fully dissociates in aqueous solution, the complete ionic equation would be:
2H+(aq) + 2Cl–(aq) + 2Na+(aq) + S2-(aq) → H2S(g) + 2Na+(aq) + 2Cl–(aq)
Removing the spectator ions we obtain the net ionic equation:
2H+(aq) + S2-(aq) → H2S(g)
Exercise 15.4e
When carbon dioxide gas combines with an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid water. Write balanced molecular, complete ionic, and net ionic equations for this process.
Check Your Answer[6]
Net Ionic Equations of Single Replacement Reactions Involving Aqueous Solutions
Recall, that during a single replacement reaction (SR) there is an exchange of elements; typically cations; an element becomes a compound and a compound becomes an element. SR reactions are always oxidation reduction (redox) reactions. These types of reactions will be discussed more in the Oxidation Reduction chapter, but we will cover the basics of writing net ionic equations for redox reactions undergoing single replacement.
Consider the following SR reaction between iron metal and copper(II) nitrate:
Balanced Molecular Equation: Fe(s) + Cu(NO3)2(aq) → Fe(NO3)2(aq) + Cu(s)
Complete Ionic Equation: Fe(s) + Cu2+(aq) + 2NO3–(aq) → Fe2+(aq) + 2NO3–(aq) + Cu(s)
Since nitrate ions are dissociated in the reactant and product side of the complete ionic equation, these are removed to produce the net ionic equation.
Net Ionic Equation: Fe(s) + Cu2+(aq) → Fe2+(aq) + Cu(s)
Exercise 15.4f
Write the complete ionic and net ionic equations for the following chemical reaction.
Ni(s) + 2HCl(aq) → NiCl2(aq) + H2(g)
Check Your Answer[7]
Net Ionic Equations of Neutralization Reactions
A neutralization reaction is a type of double displacement/replacement reaction. There is a double exchange of two cations, or two anions to form two new compounds. Neutralization reactions will be discussed more in the Acids and Bases chapter, but we will cover the basics of writing net ionic equations for neutralization reactions, now. In a neutralization reaction, the reactants are an acid and a base, and the products are often a salt (soluble or insoluble) and water, and neither reactant is the water itself:
acid + base → salt + water
Consider the following reaction between a strong acid, hydrochloric acid, and strong base, sodium hydroxide. The balanced molecular equation can be used to generate a complete ionic equation, and its net ionic equation:
Balanced Molecular Equation: HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
Complete Ionic Equation: H+(aq) + Cl–(aq) + Na+(aq) + OH–(aq) → Na+(aq) + Cl–(aq) + H2O(l)
Since nitrate ions are dissociated in the reactant and product side of the complete ionic equation, these are removed to produce the net ionic equation.
Net Ionic Equation: H+(aq) + OH–(aq) → H2O(l)
Links to Interactive Learning Tools
Explore Net Ionic Equations from the Physics Classroom.
Attribution & References
Except where otherwise noted, this page is adapted from “4.1 Writing and Balancing Chemical Equations” In CHEM 1114 – Introduction to Chemistry by Shirley Wacowich-Sgarbi and Langara Chemistry Department is licensed under CC BY-NC-SA 4.0. Adaptations and additions to content in this section were made by Jackie MacDonald for student comprehension.
- ( NH4)2S (s) → 2NH4+(aq) + S2−(aq) ↵
- Fe2(SO4)3 (s) → 2Fe3+(aq) + 3SO42−(aq) ↵
- Complete ionic equation is Ca2+(aq) + 2Cl−(aq) + Pb2+(aq) + 2NO3−(aq) → Ca2+(aq) + 2NO3−(aq) + PbCl2(s) ↵
- Ca2+(aq) and NO3−(aq) ions are spectator ions (bolded in blue), so they are cancelled. Ca2+(aq) + 2Cl−(aq) + Pb2+(aq) + 2NO3−(aq) → Ca2+(aq) + 2NO3−(aq) + PbCl2(s); The net ionic equation is Pb2+(aq) + 2Cl−(aq) → PbCl2(s) ↵
- Begin by identifying formulas for the reactants and products and arranging them properly in chemical equation form: AgNO3(aq) + CaCl2(aq) → AgCl(s) + Ca(NO3)2(aq) (unbalanced equation) Balancing this reaction results in the molecular equation for this reaction: 2AgNO3(aq) + CaCl2(aq) → 2AgCl(s) + Ca(NO3)2(aq) (balanced equation) The dissolved ionic compounds, AgNO3, CaCl2, and Ca(NO3)2, can be represented as dissociated ions to yield the complete ionic equation: 2Ag+(aq) + 2NO3−(aq) + Ca2+(aq) + 2Cl-(aq) → 2AgCl(s) + Ca2+(aq) + 2NO3−(aq) Finally, identify the spectator ion(s), in this case Ca2+(aq) and NO3−(aq), and remove them from each side of the equation to generate the net ionic equation: 2Ag+(aq) + 2Cl-(aq) → 2AgCl(s) which can be simplified to the smallest possible integers as coefficients and written as Ag+(aq) + Cl-(aq) → AgCl(s) ↵
- Begin by identifying formulas for the reactants and products and arranging them properly in chemical equation form: CO2(g) + NaOH(aq) → Na2CO3(aq) + H2O(l) (unbalanced) Balance is achieved easily in this case by changing the coefficient for NaOH to 2, resulting in the molecular equation for this reaction: CO2(g) + 2NaOH(aq) → Na2CO3(aq) + H2O(l) (balanced) The two dissolved ionic compounds, NaOH and Na2CO3, can be represented as dissociated ions to yield the complete ionic equation: CO2(g) + 2Na+(aq) +2OH-(aq) → 2Na+(aq) + CO32-(aq) + H2O(l) Finally, identify the spectator ion(s), in this case Na+(aq), and remove it from each side of the equation to generate the net ionic equation: CO2(g) + 2OH-(aq) → CO32-(aq) + H2O(l) To review a video of this solution watch How to Write the Net Ionic Equation for NaOH + CO2 = Na2CO3 + H2O (2mins 38sec). ↵
- Complete Ionic Equation: Ni(s) + 2H+(aq) + 2Cl-(aq) → Ni2+(aq) + 2Cl-(aq) + H2(g)
Since chloride ions are dissociated in both the reactant and product side of the complete ionic equation, these are removed to produce the net ionic equation.
Net Ionic Equation: Ni(s) + 2H+(aq) → Ni2+(aq) + H2(g) ↵
chemical equation in which all reactants and products are represented as neutral substances
chemical equation in which all dissolved ionic reactants and products, including spectator ions, are explicitly represented by formulas for their dissociated ions
ion that does not undergo a chemical or physical change during a reaction, but its presence is required to maintain charge neutrality
chemical equation in which only those dissolved ionic reactants and products that undergo a chemical or physical change are represented (excludes spectator ions)
A chemical reaction in which one element is substituted for another element in a compound.
reaction between an acid and a base to produce salt and water

