3.2 The Periodic Table
Learning Objectives
By the end of this section, you will be able to:
- State the periodic law and explain the organization of elements in the periodic table
- Predict the general properties of elements based on their location within the periodic table
- Identify metals, nonmetals, and metalloids by their properties and/or location on the periodic table
The Periodic Table
As early chemists worked to purify ores and discovered more elements, they realized that various elements could be grouped together by their similar chemical behaviours. One such grouping includes lithium (Li), sodium (Na), and potassium (K): These elements all are shiny, conduct heat and electricity well, and have similar chemical properties. A second grouping includes calcium (Ca), strontium (Sr), and barium (Ba), which also are shiny, good conductors of heat and electricity, and have chemical properties in common. However, the specific properties of these two groupings are notably different from each other. For example: Li, Na, and K are much more reactive than are Ca, Sr, and Ba; Li, Na, and K form compounds with oxygen in a ratio of two of their atoms to one oxygen atom, whereas Ca, Sr, and Ba form compounds with one of their atoms to one oxygen atom. Fluorine (F), chlorine (Cl), bromine (Br), and iodine (I) also exhibit similar properties to each other, but these properties are drastically different from those of any of the elements above.
Dimitri Mendeleev in Russia (1869) and Lothar Meyer in Germany (1870) independently recognized that there was a periodic relationship among the properties of the elements known at that time. Both published tables with the elements arranged according to increasing atomic mass. But Mendeleev went one step further than Meyer: He used his table to predict the existence of elements that would have the properties similar to aluminum and silicon, but were yet unknown. The discoveries of gallium (1875) and germanium (1886) provided great support for Mendeleev’s work. Although Mendeleev and Meyer had a long dispute over priority, Mendeleev’s contributions to the development of the periodic table are now more widely recognized (Figure 3.2a).
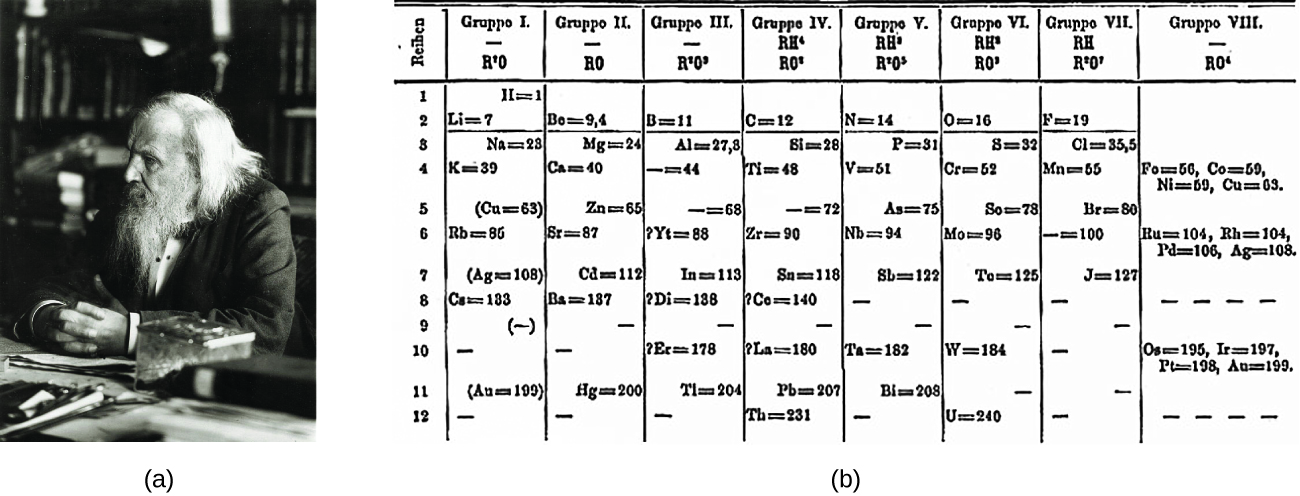
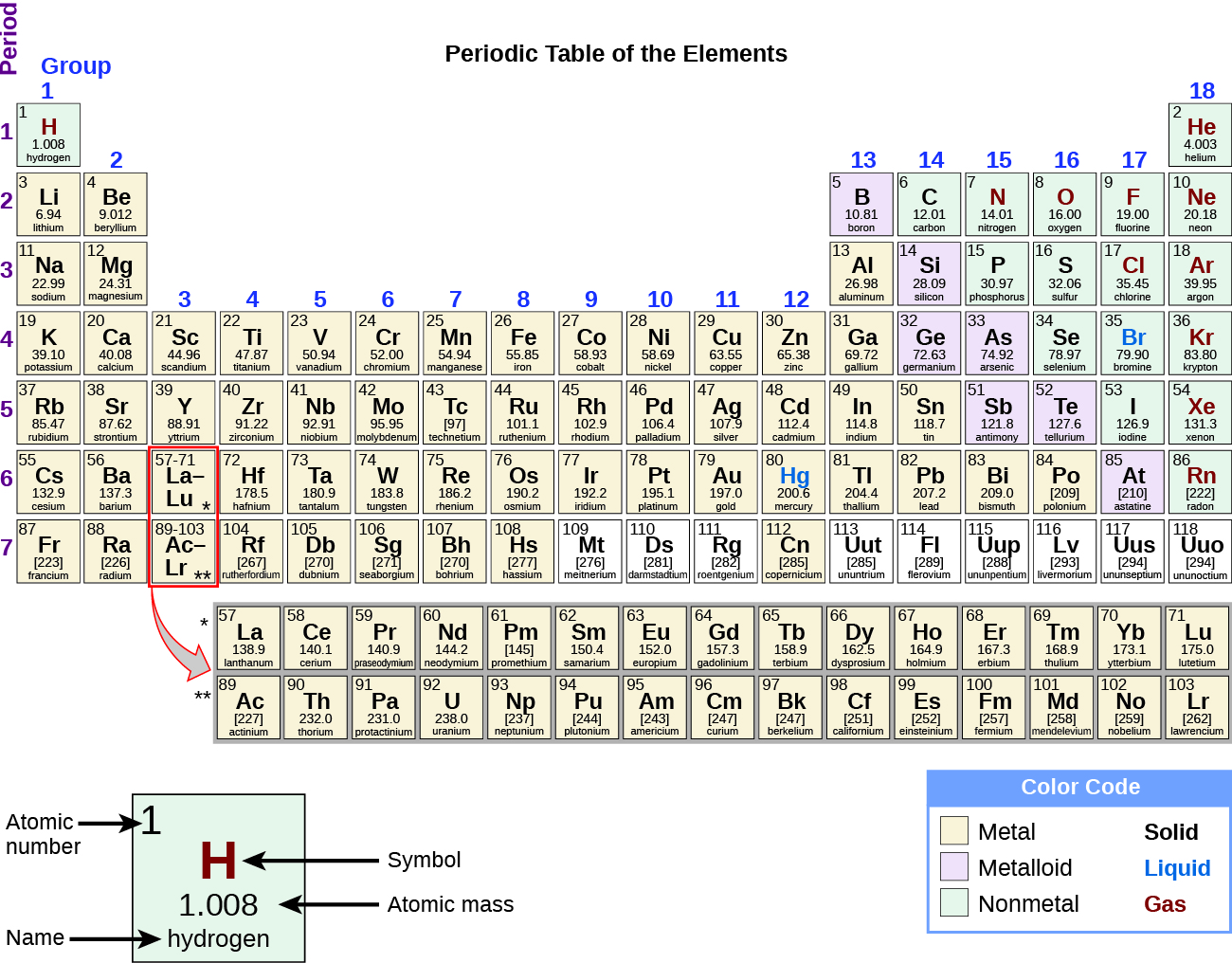
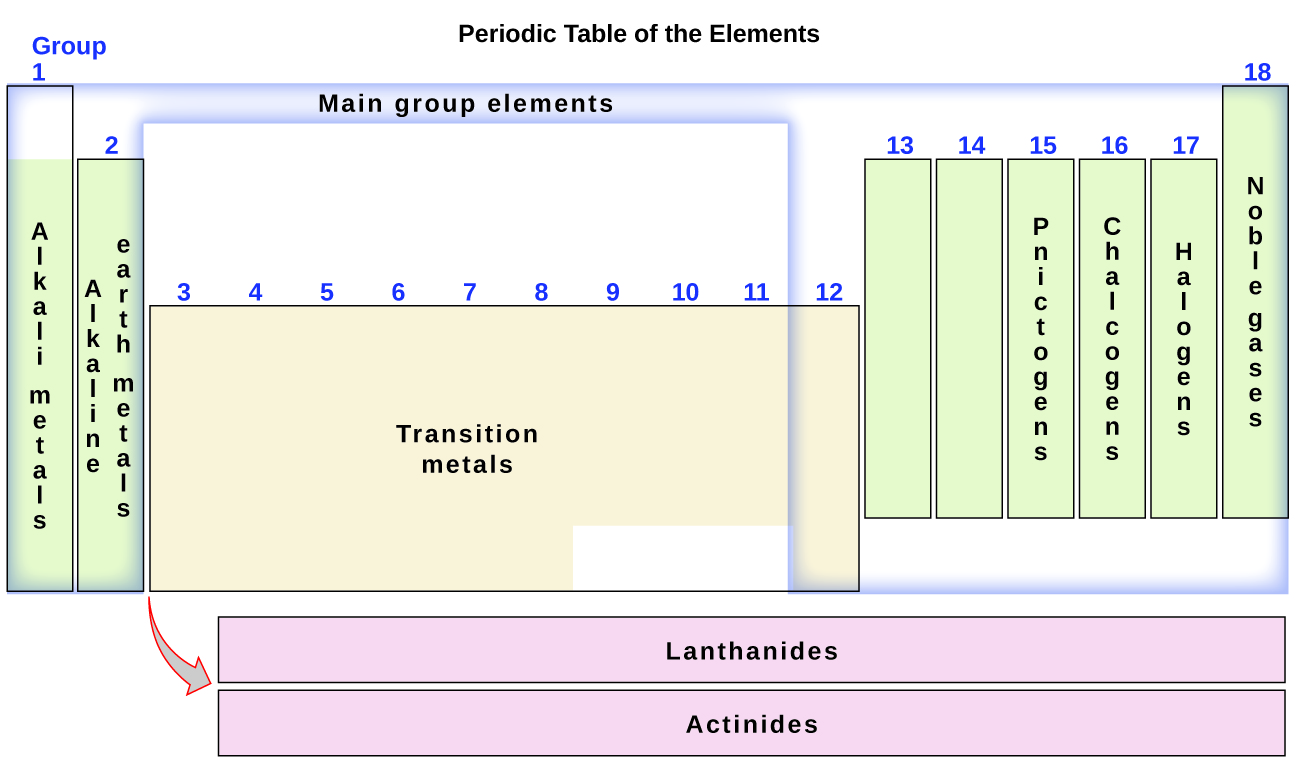
By the twentieth century, it became apparent that the periodic relationship involved atomic numbers rather than atomic masses. The modern statement of this relationship, the periodic law, is as follows: the properties of the elements are periodic functions of their atomic numbers. A modern periodic table arranges the elements in increasing order of their atomic numbers and groups atoms with similar properties in the same vertical column (Figure 3.2b). Each box represents an element and contains its atomic number, symbol, average atomic mass, and (sometimes) name. The elements are arranged in seven horizontal rows, called periods or series, and 18 vertical columns, called groups. Groups are labeled at the top of each column. In the United States, the labels traditionally were numerals with capital letters. However, IUPAC recommends that the numbers 1 through 18 be used, and these labels are more common. For the table to fit on a single page, parts of two of the rows, a total of 14 columns, are usually written below the main body of the table.
Watch The Periodic Table Song (2018 Update!) (3:04 mins)
Video source: AsapSCIENCE. (2018, February 6). The Periodic Table song (2018 Update!) [Video]. SCIENCE SONGS. YouTube.
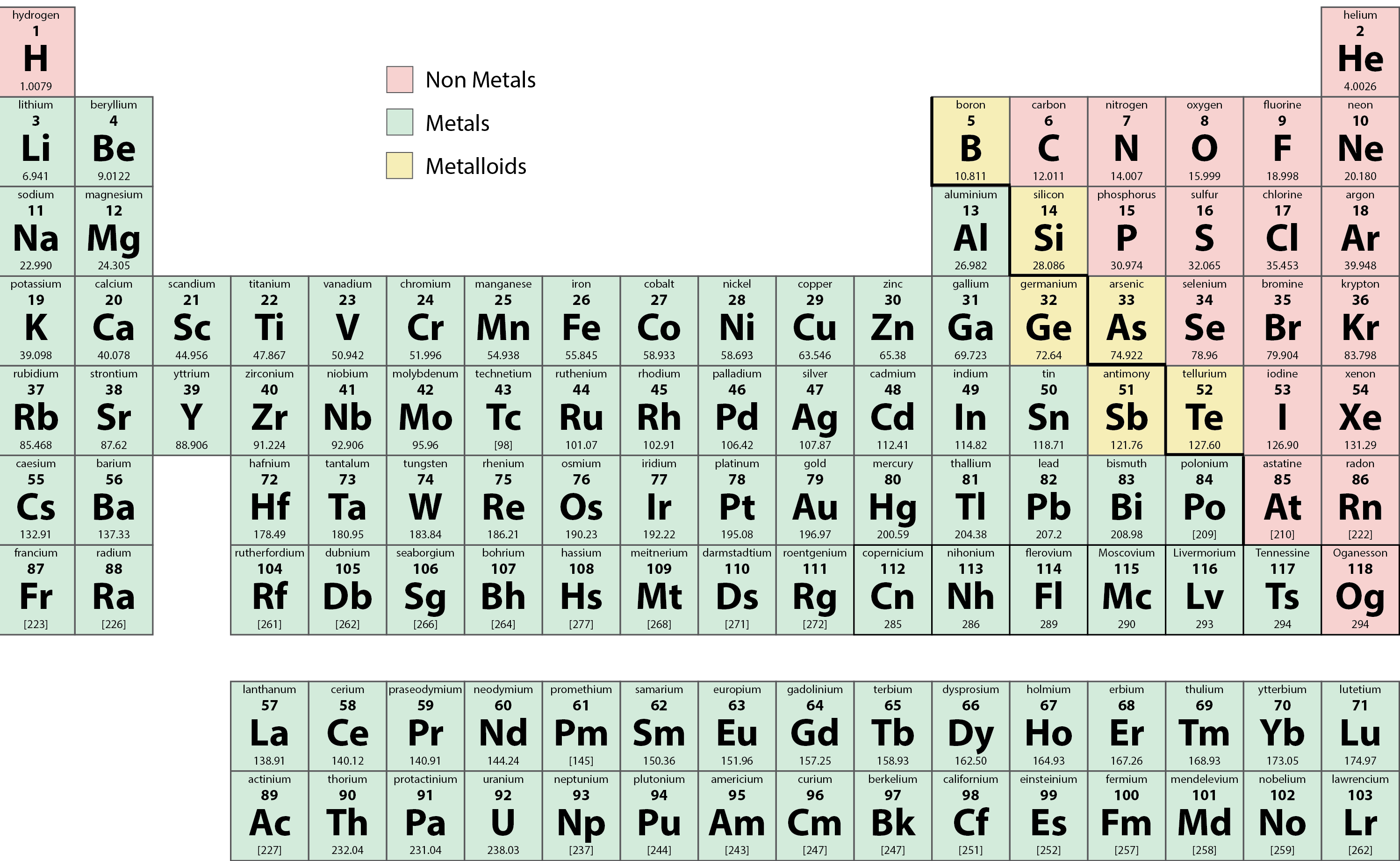
Many elements differ dramatically in their chemical and physical properties, but some elements are similar in their behaviours. For example, many elements appear shiny, are malleable (able to be deformed without breaking) and ductile (can be drawn into wires), and conduct heat and electricity well. Other elements are not shiny, malleable, or ductile, and are poor conductors of heat and electricity. We can sort the elements into large classes with common properties: metals (elements that are shiny, malleable, good conductors of heat and electricity—shaded yellow); nonmetals (elements that appear dull, poor conductors of heat and electricity—shaded green); and metalloids (elements that conduct heat and electricity moderately well, and possess some properties of metals and some properties of nonmetals—shaded purple).
Metals
Most of the representative metals do not occur naturally in an uncombined state because they readily react with water and oxygen in the air. However, it is possible to isolate elemental beryllium, magnesium, zinc, cadmium, mercury, aluminum, tin, and lead from their naturally occurring minerals and use them because they react very slowly with air. Part of the reason why these elements react slowly is that these elements react with air to form a protective coating. The formation of this protective coating is passivation. The coating is a nonreactive film of oxide or some other compound. Elemental magnesium, aluminum, zinc, and tin are important in the fabrication of many familiar items, including wire, cookware, foil, and many household and personal objects. Although beryllium, cadmium, mercury, and lead are readily available, there are limitations in their use because of their toxicity.
Mercury – An Interesting Metal
Mercury is the only metal that is liquid at 25 °C. Many metals dissolve in mercury, forming solutions called amalgams (see the feature on Amalgams), which are alloys of mercury with one or more other metals. Mercury, shown in Figure 3.2c, is a nonreactive element that is more difficult to oxidize than hydrogen. Thus, it does not displace hydrogen from acids; however, it will react with strong oxidizing acids, such as nitric acid:
The clear NO initially formed quickly undergoes further oxidation to the reddish brown NO2.

Amalgams
An amalgam is an alloy of mercury with one or more other metals. This is similar to considering steel to be an alloy of iron with other metals. Most metals will form an amalgam with mercury, with the main exceptions being iron, platinum, tungsten, and tantalum.
Due to toxicity issues with mercury, there has been a significant decrease in the use of amalgams. Historically, amalgams were important in electrolytic cells and in the extraction of gold. Amalgams of the alkali metals still find use because they are strong reducing agents and easier to handle than the pure alkali metals.
Prospectors had a problem when they found finely divided gold. They learned that adding mercury to their pans collected the gold into the mercury to form an amalgam for easier collection. Unfortunately, losses of small amounts of mercury over the years left many streams in California polluted with mercury.
Dentists use amalgams containing silver and other metals to fill cavities. There are several reasons to use an amalgam including low cost, ease of manipulation, and longevity compared to alternate materials. Dental amalgams are approximately 50% mercury by weight, which, in recent years, has become a concern due to the toxicity of mercury.
After reviewing the best available data, the Food and Drug Administration (FDA) considers amalgam-based fillings to be safe for adults and children over six years of age. Even with multiple fillings, the mercury levels in the patients remain far below the lowest levels associated with harm. Clinical studies have found no link between dental amalgams and health problems. Health issues may not be the same in cases of children under six or pregnant women. The FDA conclusions are in line with the opinions of the Environmental Protection Agency (EPA) and Centres for Disease Control (CDC). The only health consideration noted is that some people are allergic to the amalgam or one of its components.
Nonmetals
The nonmetals are elements located in the upper right portion of the periodic table. Their properties and behaviour are quite different from those of metals on the left side. Under normal conditions, more than half of the nonmetals are gases, one is a liquid, and the rest include some of the softest and hardest of solids. The nonmetals exhibit a rich variety of chemical behaviours. They include the most reactive and least reactive of elements, and they form many different ionic and covalent compounds.
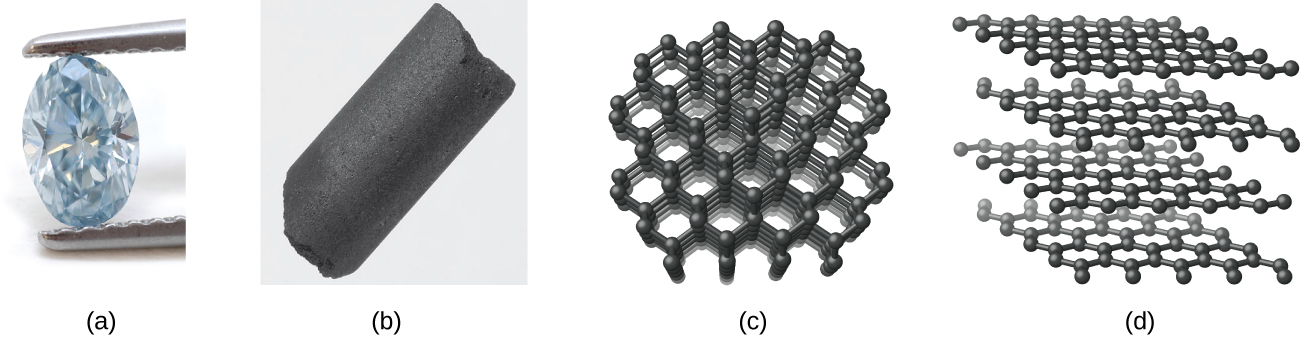
Carbon – An Interesting Nonmetal
Carbon occurs in the uncombined (elemental) state in many forms, such as diamond, graphite, charcoal, coke, carbon black, graphene, and fullerene.
Diamond, shown in Figure 3.2d, is a very hard crystalline material that is colourless and transparent when pure. Each atom forms four single bonds to four other atoms at the corners of a tetrahedron (sp3 hybridization); this makes the diamond a giant molecule. Carbon-carbon single bonds are very strong, and, because they extend throughout the crystal to form a three-dimensional network, the crystals are very hard and have high melting points (~4400 °C).
Metalloids
A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. The metalloids are boron, silicon, germanium, arsenic, antimony, and tellurium. These elements look metallic; however, they do not conduct electricity as well as metals so they are semiconductors. They are semiconductors because their electrons are more tightly bound to their nuclei than are those of metallic conductors. Their chemical behaviour falls between that of metals and nonmetals. For example, the pure metalloids form covalent crystals like the nonmetals, but like the metals, they generally do not form monatomic anions. This intermediate behaviour is in part due to their intermediate electronegativity values.
Boron and Silicon – Two Interesting Metalloids
Boron constitutes less than 0.001% by weight of the earth’s crust. In nature, it only occurs in compounds with oxygen. Boron is widely distributed in volcanic regions as boric acid, B(OH)3, and in dry lake regions, including the desert areas of California, as borates and salts of boron oxyacids, such as borax, Na2B4O7⋅10H2O. Silicon makes up nearly one-fourth of the mass of the earth’s crust—second in abundance only to oxygen. Extremely pure silicon is necessary for the manufacture of semiconductor electronic devices.
Boron burns at 700 °C in oxygen, forming boric oxide, B2O3. Boric oxide is necessary for the production of heat-resistant borosilicate glass, like that shown in Figure 3.2e and certain optical glasses. Boric oxide dissolves in hot water to form boric acid, B(OH)3:

When silicon reacts with oxygen, silicon dioxide is formed. Silicon dioxide, silica, occurs in both crystalline and amorphous forms. The usual crystalline form of silicon dioxide is quartz, a hard, brittle, clear, colourless solid. It is useful in many ways—for architectural decorations, semiprecious jewels, and frequency control in radio transmitters. Silica takes many crystalline forms, or polymorphs, in nature. Trace amounts of Fe3+ in quartz give amethyst its characteristic purple colour. The term quartz is also used for articles such as tubing and lenses that are manufactured from amorphous silica. Opal is a naturally occurring form of amorphous silica.
The Organization of the Periodic Table
The elements can also be classified into the main-group elements (or representative elements) in the columns labeled 1, 2, and 13–18; the transition metals in the columns labeled 3–12; and inner transition metals in the two rows at the bottom of the table (the top-row elements are called lanthanides and the bottom-row elements are actinides; Figure 3.2f). The elements can be subdivided further by more specific properties, such as the composition of the compounds they form. For example, the elements in group 1 (the first column) form compounds that consist of one atom of the element and one atom of hydrogen. These elements (except hydrogen) are known as alkali metals, and they all have similar chemical properties. The elements in group 2 (the second column) form compounds consisting of one atom of the element and two atoms of hydrogen: These are called alkaline earth metals, with similar properties among members of that group. Other groups with specific names are the pnictogens (group 15), chalcogens (group 16), halogens (group 17), and the noble gases (group 18, also known as inert gases). The groups can also be referred to by the first element of the group: For example, the chalcogens can be called the oxygen group or oxygen family. Hydrogen is a unique, nonmetallic element with properties similar to both group 1A and group 7A elements. For that reason, hydrogen may be shown at the top of both groups, or by itself.
Group 1: The Alkali Metals
The alkali metals lithium, sodium, potassium, rubidium, cesium, and francium constitute group 1 of the periodic table. Although hydrogen is in group 1, it is a nonmetal. The name alkali metal is in reference to the fact that these metals and their oxides react with water to form very basic (alkaline) solutions.
The properties of the alkali metals are similar to each other as expected for elements in the same family. The alkali metals have the largest atomic radii and the lowest first ionization energy in their periods. This combination makes it very easy to remove the single electron in the outermost (valence) shell of each. The easy loss of this valence electron means that these metals readily form stable cations with a charge of 1+. Their reactivity increases with the increasing atomic number due to the ease of losing the lone valence electron (decreasing ionization energy).
The alkali metals all react vigorously with water to form hydrogen gas and a basic solution of the metal hydroxide.
Group 2: The Alkaline Earth Metals
The alkaline earth metals (beryllium, magnesium, calcium, strontium, barium, and radium) constitute group 2 of the periodic table. The name alkaline metal comes from the fact that the oxides of the heavier members of the group react with water to form alkaline solutions. The nuclear charge increases when going from group 1 to group 2. Because of this charge increase, the atoms of the alkaline earth metals are smaller and have higher first ionization energies than the alkali metals within the same period. The higher ionization energy makes the alkaline earth metals less reactive than the alkali metals; however, they are still very reactive elements. Their reactivity increases, as expected, with increasing size and decreasing ionization energy. In chemical reactions, these metals readily lose both valence electrons to form compounds in which they exhibit an oxidation state of 2+.
Groups 7 and 8: Halogens and Noble Gases
The noble gases are all monatomic, whereas the other nonmetal gases—hydrogen, nitrogen, oxygen, fluorine, and chlorine—normally exist as the diatomic molecules H2, N2, O2, F2, and Cl2. The other halogens are also diatomic; Br2 is a liquid and I2 exists as a solid under normal conditions.
Halogens
Halogens are found in group 17 (or 7A) within the periodic table. These include Fluorine, Chlorine, Bromine and Iodine. Fluorine is a pale yellow gas, chlorine is a greenish-yellow gas, bromine is a deep reddish-brown liquid, and iodine is a grayish-black crystalline solid. Liquid bromine has a high vapour pressure, and the reddish vapour is readily visible in Figure 3.2g. Iodine crystals have a noticeable vapour pressure. When gently heated, these crystals sublime and form a beautiful deep violet vapour.

The Noble Gases
The elements in group 18 (or 8A) are the noble gases (helium, neon, argon, krypton, xenon, and radon). They earned the name “noble” because they were assumed to be nonreactive since they filled valence shells. In 1962, Dr. Neil Bartlett at the University of British Columbia proved this assumption to be false.
These elements are present in the atmosphere in small amounts. Some natural gas contains 1–2% helium by mass. Helium is isolated from natural gas by liquefying the condensable components, leaving only helium as a gas. The United States possesses most of the world’s commercial supply of this element in its helium-bearing gas fields. Argon, neon, krypton, and xenon come from the fractional distillation of liquid air. Radon comes from other radioactive elements. More recently, it was observed that this radioactive gas is present in very small amounts in soils and minerals. Its accumulation in well-insulated, tightly sealed buildings, however, constitutes a health hazard, primarily lung cancer.
Exercise 3.2a
Check Your Learning Exercise (Text Version)
Atoms of elements are essential for life. Identify the group name (Alkali Metal, Gas, Chalcogen, Alkaline Earth Metal, Halogen) that each of the following elements belong to:
- chlorine belongs to
- calcium and barium belongs to
- sodium and lithium belongs to
- sulfur and selenium belongs to
- krypton belongs to
Check Your Answer[1]
Source: “Exercise 3.1a” is adapted from “3.6 The Periodic Table Example 1” from General Chemistry 1 & 2, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson, licensed under CC BY 4.0.
In studying the periodic table, you might have noticed something about the atomic masses of some of the elements. Element 43 (technetium), element 61 (promethium), and most of the elements with atomic number 84 (polonium) and higher have their atomic mass given in square brackets. This is done for elements that consist entirely of unstable, radioactive isotopes (you will learn more about radioactivity in the nuclear chemistry chapter). An average atomic weight cannot be determined for these elements because their radioisotopes may vary significantly in relative abundance, depending on the source, or may not even exist in nature. The number in square brackets is the atomic mass number (and approximate atomic mass) of the most stable isotope of that element.
Links to Interactive Learning Tools
Click on the interactive periodic table, from the Royal Society of Chemistry, which you can use to explore the properties of the elements (includes podcasts and videos of each element).
Classify Metals, Metalloids and Nonmetals from the Physics Classroom.
Explore the Classification of Matter from the Physics Classroom.
Explore Elements, Atoms, and Ions Practice from eCampusOntario H5P Studio.
Attribution & References
- (a) Halogen; (b) Alkaline Earth Metal; (c) Alkali Metal; (d) Chalcogen; (e) Gas ↵
properties of the elements are periodic function of their atomic numbers.
table of the elements that places elements with similar chemical properties close together
(also, series) horizontal row of the periodic table
(also, period) horizontal row of the period table
vertical column of the periodic table
element that is shiny, malleable, good conductor of heat and electricity
element that appears dull, poor conductor of heat and electricity
element that conducts heat and electricity moderately well, and possesses some properties of metals and some properties of nonmetals
It is a widely-used metal finishing process to prevent corrosion
the ability of a substance to crystallize into different crystalline forms
(also, representative element) element in columns 1, 2, and 12–18
(also, main-group element) element in columns 1, 2, and 12–18
element in columns 3–11
(also, lanthanide or actinide) element in the bottom two rows; if in the first row, also called lanthanide, or if in the second row, also called actinide
inner transition metal in the top of the bottom two rows of the periodic table
Inner transition metal in the bottom of the bottom two rows of the periodic table
element in group 1
element in group 2
element in group 15
chalcogen
element in group 17
(also, inert gas) element in group 18
(also, noble gas) element in group 18
element in group 16

