8.3 Classifying and Completing Single- and Double-Displacement Reactions
Learning Objectives
By the end of this section, you will be able to:
- Identify chemical reactions as single-replacement reactions and double-replacement reactions.
- Use the periodic table, an activity series, or solubility rules to predict whether single-replacement reactions or double-replacement reactions will occur.
Up to now, we have presented chemical reactions as a topic, but we have not discussed how the products of a chemical reaction can be predicted. Here we will begin our study of certain types of chemical reactions that allow us to predict what the products of the reaction will be.
A single-replacement reaction is a chemical reaction in which one element is substituted for another element in a compound, generating a new element and a new compound as products. For example:
2HCl(aq) + Zn(s) → ZnCl2(aq) + H2(g)
is an example of a single-replacement reaction. The hydrogen atoms in HCl are replaced by Zn atoms, and in the process a new element—hydrogen—is formed. Another example of a single-replacement reaction is:
2NaCl(aq) + F2(g) → 2NaF(s) + Cl2(g)
Here the negatively charged ion changes from chloride to fluoride. A typical characteristic of a single-replacement reaction is that there is one element as a reactant and another element as a product.
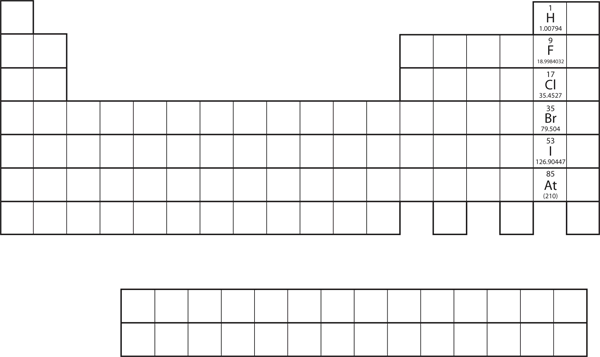
Not all proposed single-replacement reactions will occur between two given reactants. This is most easily demonstrated with fluorine, chlorine, bromine, and iodine. Collectively, these elements are called the halogens and are in the next-to-last column on the periodic table (see Figure 8.3a "Halogens on the Periodic Table"). The elements on top of the column will replace the elements below them on the periodic table but not the other way around. Thus, the reaction represented by:
CaI2(s) + Cl2(g) → CaCl2(s) + I2(s)
This reaction will occur, but the reaction
CaF2(s) + Br2(ℓ) → CaBr2(s) + F2(g)
will not because bromine is below fluorine on the periodic table. This is just one of many ways the periodic table helps us understand chemistry.
Example 8.3a
Problems
Will a single-replacement reaction occur? If so, identify the products.
- MgCl2 + I2 → ?
- CaBr2 + F2 → ?
Solutions
- Because iodine is below chlorine on the periodic table, a single-replacement reaction will not occur.
- Because fluorine is above bromine on the periodic table, a single-replacement reaction will occur, and the products of the reaction will be CaF2 and Br2.
Exercise 8.3a
Will a single-replacement reaction occur? If so, identify the products.
FeI2 + Cl2 → ?
Check Your Answer[1]
Chemical reactivity trends are easy to predict when replacing anions in simple ionic compounds—simply use their relative positions on the periodic table. However, when replacing the cations, the trends are not as straightforward. This is partly because there are so many elements that can form cations; an element in one column on the periodic table may replace another element nearby, or it may not. A list called the activity series does the same thing the periodic table does for halogens: it lists the elements that will replace elements below them in single-replacement reactions. A simple activity series is shown below.
Activity Series for Cation Replacement in Single-Replacement Reactions
- Li
- K
- Ba
- Sr
- Ca
- Na
- Mg
- Al
- Mn
- Zn
- Cr
- Fe
- Ni
- Sn
- Pb
- H2
- Cu
- Hg
- Ag
- Pd
- Pt
- Au
An element with a lower number will replace an element with a higher number in compounds undergoing a single-replacement reaction. Elements will not replace elements with a lower number in compounds. In many sources, these elements are listed as a long vertical list (not numbered).
Using the activity series is similar to using the positions of the halogens on the periodic table. An element on top will replace an element below it in compounds undergoing a single-replacement reaction. Elements will not replace elements above them in compounds.
Example 8.3b
Problems
Use the activity series to predict the products, if any, of each equation.
- FeCl2 + Zn → ?
- HNO3 + Au → ?
Solutions
- Because zinc is above iron in the activity series, it will replace iron in the compound. The products of this single-replacement reaction are ZnCl2 and Fe.
- Gold is below hydrogen in the activity series. As such, it will not replace hydrogen in a compound with the nitrate ion. No reaction is predicted.
Exercise 8.3b
Use the activity series to predict the products, if any, of this equation.
AlPO4 + Mg → ?
Check Your Answer[2]
A double-replacement reaction occurs when parts of two ionic compounds are exchanged, making two new compounds. A characteristic of a double-replacement equation is that there are two compounds as reactants and two different compounds as products.
Evidence of a double replacement reaction are the following:
- The evolution of heat
- The formation of an insoluble precipitate
- The production of gas bubbles
Source: (Hein et al., 2013, p. 153)
An example of a double replacement reaction is:
CuCl2(aq) + 2AgNO3(aq) → Cu(NO3)2(aq) + 2AgCl(s)
There are two equivalent ways of considering a double-replacement equation: either the cations are swapped, or the anions are swapped. (You cannot swap both; you would end up with the same substances you started with.) Either perspective should allow you to predict the proper products, as long as you pair a cation with an anion and not a cation with a cation or an anion with an anion.
Example 8.3c
Problem
Predict the products of this double-replacement equation: BaCl2 + Na2SO4 → ?
Solution
Thinking about the reaction as either switching the cations or switching the anions, we would expect the products to be BaSO4 and NaCl.
Exercise 8.3c
Predict the products of this double-replacement equation: KBr + AgNO3 → ?
Check Your Answer[3]
Predicting whether a double-replacement reaction occurs is somewhat more difficult than predicting a single-replacement reaction. However, there is one type of double-replacement reaction that we can predict: the precipitation reaction. A precipitation reaction occurs when two ionic compounds are dissolved in water and form a new ionic compound that does not dissolve; this new compound falls out of solution as a solid precipitate. The formation of a solid precipitate is the driving force that makes the reaction proceed.
For example, consider the double-replacement reaction between Na2SO4 and SrCl2. The double-replacement reaction products are NaCl and SrSO4. The balanced chemical equation is:
Na2SO4(aq) + SrCl2(aq) → 2NaCl(aq) + SrSO4(s)
You would expect to see a visual change corresponding to SrSO4 precipitating out of solution as noted by the (s) in the product formed within the chemical equation (Figure 8.3b "Double-Replacement Reactions").

General examples of double replacement reactions include:
- Neutralization of an Acid and a Base (heat is released by the production of water)
Example: [latex]\ce{HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (l)} [/latex]
- Metal Oxide + Acid (heat is released by the production of water)
Example: [latex]\ce{CuO + 2HNO3 (aq) → Cu(NO3)2 (aq) + H2O (l) }[/latex]
- Formation of an Insoluble Precipitate (precipitate is formed as indicated by the (s) in the product)
Example: [latex]\ce{BaCl2 (aq) + 2AgNO3 (aq) → 2AgCl (s) + Ba(NO3)2 (aq) }[/latex]
- Formation of a Gas ( a gas is formed as indicated by the (g) in the product).
Example: [latex]\ce{H2SO4 (aq) + NaCl (s) → NaHSO4 (s) + HCl (g) }[/latex]
Source: (Hein et al., 2013, p. 153)
Example 8.3d
Problem
What are the products of the double-replacement reaction
- NaOH + FeCl2 → ?
Solution
- The balanced chemical equation is: 2NaOH(aq) + FeCl2(aq) → 2NaCl(aq) + Fe(OH)2(s)
Exercise 8.3d
Check Your Learning Exercise (Text Version)
For the following reactions, classify the reaction as one of the following: single displacement, decomposition, combination, double displacement, or combustion.
- 2CH3OH (g) + 3O2 (g) → 2CO2 (g) + 4H2O (g)
- 2H2O (l) → 2H2 (g) + O2 (g)
- P4 (s) + 5O2 (g) → P4O10 (s)
- 2Al (s) + 3H2SO4 (aq) → Al2(SO4)3 (s) + 3H2 (g)
- ZnS (s) + 2HCl (aq) → ZnCl2 (aq) + H2S (g)
Check Your Answer[4]
Source: "Exercise 8.3d" by Adrienne Richards is adapted from "7.2 Classifying Chemical Reactions" from General Chemistry 1 & 2, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson, licensed under CC BY 4.0.
Indigenous Perspective: Natural Chemical Reactions

Chemical reactions related to Indigenous practices in Canada are used as a teaching tool, described within the document "Chemical Reactions: Background Information Science 10".
The teaching tool was developed in collaboration with the Steward Resources Center, the Government of Saskatchewan and the Saskatchewan Teachers' Federation.
Chemical reactions are involved in practices passed down from one generation to the next. This is important so that First Nations and Metis communities can make their own supplies by following the methods from previous generations. These practices include the process of making natural bleach, jellies, medicines, meals and household products.
Links to Interactive Learning Tools
Practice Chemical Reaction Types from the Physics Classroom.
Practice Writing Chemical Equations from the Physics Classroom.
Attribution & References
Except where otherwise noted, this page is adapted by Adrienne Richards from "Chapter 4: Chemical Reactions and Equations: Types of Chemical Reactions: Single and Double-Displacement Reactions" In Introductory Chemistry: 1st Canadian Edition by David W. Ball and Jessica A. Key, licensed under CC BY-NC-SA 4.0.
Reference
Hein, M., Pattison, S., Arena, S., & Best, L. (2013). Introduction to general, organic, and biochemistry (11th ed.). John Wiley & Sons, Inc.
A chemical reaction in which one element is substituted for another element in a compound.
A list of elements that will replace elements below them in single-replacement reactions.
A chemical reaction in which parts of two ionic compounds are exchanged.
reaction that produces one or more insoluble products; when reactants are ionic compounds, sometimes called double-displacement or metathesis
insoluble product that forms from reaction of soluble reactants

