17.7 Relative Strengths of Acids and Bases
Learning Objectives
- Assess the relative strengths of acids and bases according to their ionization constants
- Rationalize trends in acid–base strength in relation to molecular structure
- Carry out equilibrium calculations for weak acid–base systems
We can rank the strengths of acids by the extent to which they ionize in aqueous solution. The reaction of an acid with water is given by the general expression:
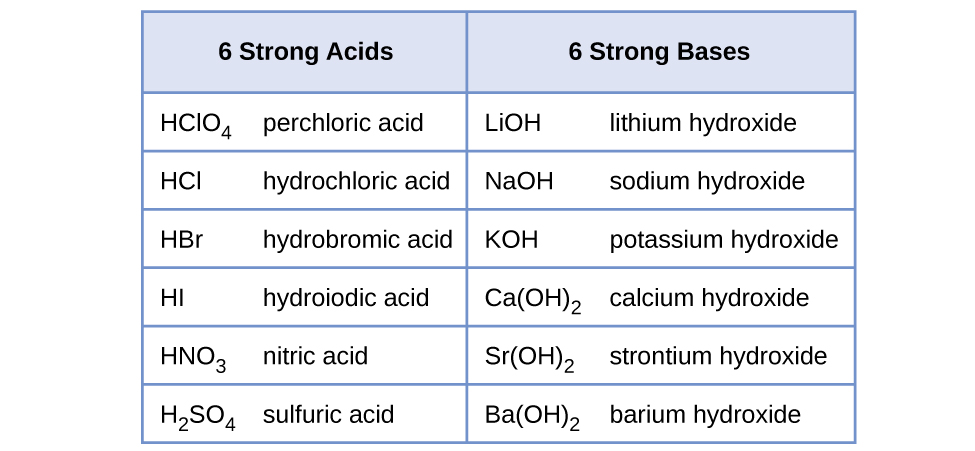
Water is the base that reacts with the acid HA, A− is the conjugate base of the acid HA, and the hydronium ion is the conjugate acid of water. A strong acid yields 100% (or very nearly so) of H3O+ and A− when the acid ionizes in water; Figure 17.7a lists several strong acids. A weak acid gives small amounts of H3O+ and A−.
The relative strengths of acids may be determined by measuring their equilibrium constants in aqueous solutions. In solutions of the same concentration, stronger acids ionize to a greater extent, and so yield higher concentrations of hydronium ions than do weaker acids. The equilibrium constant for an acid is called the acid-ionization constant, Ka. For the reaction of an acid HA:
we write the equation for the ionization constant as:
where the concentrations are those at equilibrium. Although water is a reactant in the reaction, it is the solvent as well, so we do not include [H2O] in the equation. The larger the Ka of an acid, the larger the concentration of H3O+ and A− relative to the concentration of the nonionized acid, HA. Thus a stronger acid has a larger ionization constant than does a weaker acid. The ionization constants increase as the strengths of the acids increase.
Another measure of the strength of an acid is its percent ionization. The percent ionization of a weak acid is the ratio of the concentration of the ionized acid to the initial acid concentration, times 100:
Because the ratio includes the initial concentration, the percent ionization for a solution of a given weak acid varies depending on the original concentration of the acid, and actually decreases with increasing acid concentration.
Example 17.7a
Calculation of Percent Ionization from pH
Calculate the percent ionization of a 0.125-M solution of nitrous acid (a weak acid), with a pH of 2.09.
Solution
The percent ionization for an acid is:
The chemical equation for the dissociation of the nitrous acid is: [latex]\text{HNO}_2(aq)\;+\;\text{H}_2\text{O}(l)\;{\rightleftharpoons}\;\text{NO}_2^{\;\;-}(aq)\;+\;\text{H}_3\text{O}^{+}(aq)[/latex].
Since [latex]10^{-\text{pH}} = [\text{H}_3\text{O}^{+}][/latex], we find that 10−2.09 = 8.1 × 10−3M, so that percent ionization is:
Remember, the logarithm 2.09 indicates a hydronium ion concentration with only two significant figures.
Exercise 17.7a
Calculate the percent ionization of a 0.10-M solution of acetic acid with a pH of 2.89..
Check Your Answer[1]
We can rank the strengths of bases by their tendency to form hydroxide ions in aqueous solution. The reaction of a Brønsted-Lowry base with water is given by:
Water is the acid that reacts with the base, HB+ is the conjugate acid of the base B, and the hydroxide ion is the conjugate base of water. A strong base yields 100% (or very nearly so) of OH− and HB+ when it reacts with water; Figure 17.7a lists several strong bases. A weak base yields a small proportion of hydroxide ions. Soluble ionic hydroxides such as NaOH are considered strong bases because they dissociate completely when dissolved in water.
Exercise 17.7b
Practice using the following PhET simulation: Acid and Base Solutions
As we did with acids, we can measure the relative strengths of bases by measuring their base-ionization constant (Kb) in aqueous solutions. In solutions of the same concentration, stronger bases ionize to a greater extent, and so yield higher hydroxide ion concentrations than do weaker bases. A stronger base has a larger ionization constant than does a weaker base. For the reaction of a base, B:
we write the equation for the ionization constant as:
where the concentrations are those at equilibrium. Again, we do not include [H2O] in the equation because water is the solvent. The chemical reactions and ionization constants of the three bases shown are:
As with acids, percent ionization can be measured for basic solutions, but will vary depending on the base ionization constant and the initial concentration of the solution.
Consider the ionization reactions for a conjugate acid-base pair, HA − A−:
Adding these two chemical equations yields the equation for the autoionization for water:
As shown in the previous chapter on equilibrium, the K expression for a chemical equation derived from adding two or more other equations is the mathematical product of the other equations’ K expressions. Multiplying the mass-action expressions together and cancelling common terms, we see that:
For example, the acid ionization constant of acetic acid (CH3COOH) is 1.8 × 10−5, and the base ionization constant of its conjugate base, acetate ion (CH3COO–), is 5.6 × 10−10. The product of these two constants is indeed equal to Kw:
The extent to which an acid, HA, donates protons to water molecules depends on the strength of the conjugate base, A−, of the acid. If A− is a strong base, any protons that are donated to water molecules are recaptured by A−. Thus there is relatively little A− and H3O+ in solution, and the acid, HA, is weak. If A− is a weak base, water binds the protons more strongly, and the solution contains primarily A− and H3O+—the acid is strong. Strong acids form very weak conjugate bases, and weak acids form stronger conjugate bases (Figure 17.7b).
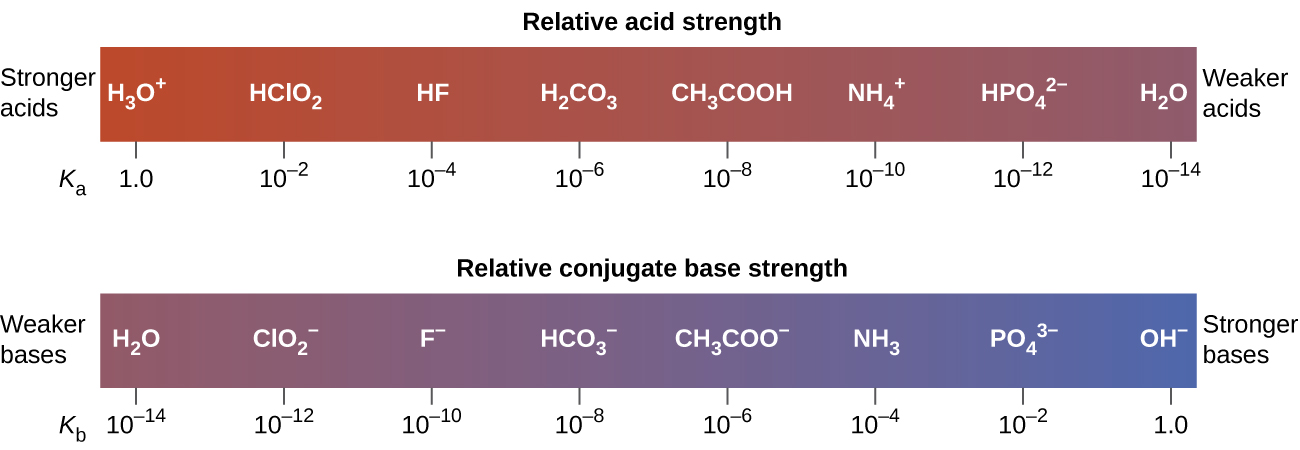
Figure 17.7c lists a series of acids and bases in order of the decreasing strengths of the acids and the corresponding increasing strengths of the bases. The acid and base in a given row are conjugate to each other.
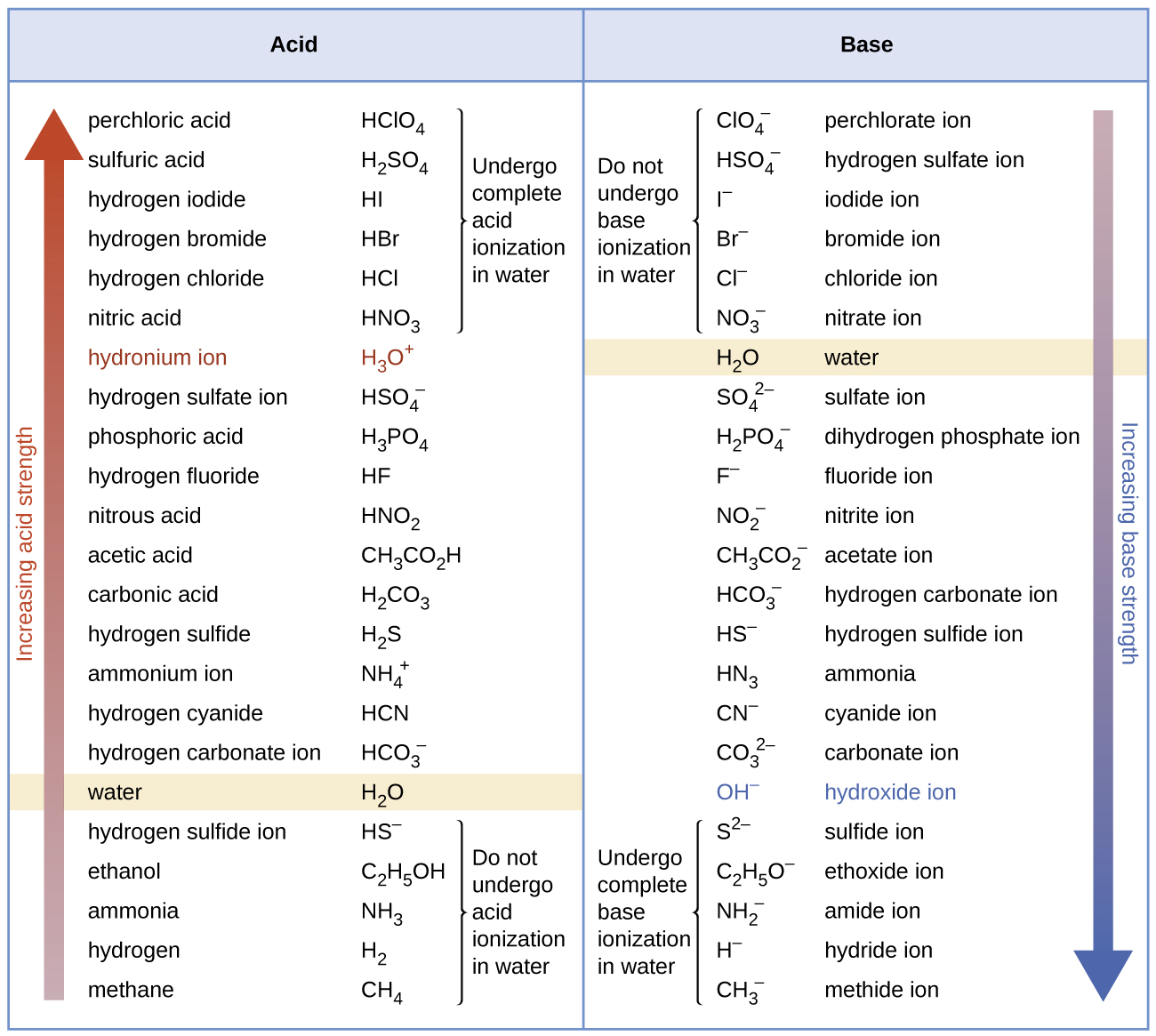
The first six acids in Figure 17.7c are the most common strong acids. These acids are completely dissociated in aqueous solution. The conjugate bases of these acids are weaker bases than water. When one of these acids dissolves in water, their protons are completely transferred to water, the stronger base.
Those acids that lie between the hydronium ion and water in Figure 17.7c form conjugate bases that can compete with water for possession of a proton. Both hydronium ions and nonionized acid molecules are present in equilibrium in a solution of one of these acids. Compounds that are weaker acids than water (those found below water in the column of acids) in Figure 17.7c exhibit no observable acidic behaviour when dissolved in water. Their conjugate bases are stronger than the hydroxide ion, and if any conjugate base were formed, it would react with water to re-form the acid.
The extent to which a base forms hydroxide ion in aqueous solution depends on the strength of the base relative to that of the hydroxide ion, as shown in the last column in Figure 17.7c. A strong base, such as one of those lying below the hydroxide ion, accepts protons from water to yield 100% of the conjugate acid and hydroxide ion. Those bases lying between water and hydroxide ion accept protons from water, but a mixture of the hydroxide ion and the base results. Bases that are weaker than water (those that lie above water in the column of bases) show no observable basic behaviour in aqueous solution.
Example 17.7b
The Product Ka × Kb = Kw
Use the Kb for the nitrite ion, NO2–, to calculate the Ka for its conjugate acid.
Solution
Kb for NO2– is given in this section as 2.17 × 10−11. The conjugate acid of NO2– is HNO2; Ka for HNO2 can be calculated using the relationship:
Solving for Ka, we get:
This answer can be verified by finding the Ka for HNO2 in Appendix I.
Exercise 17.7c
We can determine the relative acid strengths of NH4+ and HCN by comparing their ionization constants. The ionization constant of HCN is given in Appendix I as 4.9 × 10−10. The ionization constant of NH4+ is not listed, but the ionization constant of its conjugate base, NH3, is listed as 1.8 × 10−5. Determine the ionization constant of NH4+, and decide which is the stronger acid, HCN or NH4+.
Check Your Answer[2]
The Ionization of Weak Acids and Weak Bases
Many acids and bases are weak; that is, they do not ionize fully in aqueous solution. A solution of a weak acid in water is a mixture of the nonionized acid, hydronium ion, and the conjugate base of the acid, with the nonionized acid present in the greatest concentration. Thus, a weak acid increases the hydronium ion concentration in an aqueous solution (but not as much as the same amount of a strong acid).
Acetic acid, CH3CO2H, is a weak acid. When we add acetic acid to water, it ionizes to a small extent according to the equation:
giving an equilibrium mixture with most of the acid present in the nonionized (molecular) form. This equilibrium, like other equilibria, is dynamic; acetic acid molecules donate hydrogen ions to water molecules and form hydronium ions and acetate ions at the same rate that hydronium ions donate hydrogen ions to acetate ions to reform acetic acid molecules and water molecules. We can tell by measuring the pH of an aqueous solution of known concentration that only a fraction of the weak acid is ionized at any moment (Figure 17.7d). The remaining weak acid is present in the nonionized form.
For acetic acid, at equilibrium:

| Ionization Reaction | Ka at 25 °C |
|---|---|
| [latex]\text{HSO}_4^{\;\;-}\;+\;\text{H}_2\text{O}\;{\rightleftharpoons}\;\text{H}_3\text{O}^{+}\;+\;\text{SO}_4^{\;\;2-}[/latex] | 1.2 × 10−2 |
| [latex]\text{HF}\;+\;\text{H}_2\text{O}\;{\rightleftharpoons}\;\text{H}_3\text{O}^{+}\;+\;\text{F}^{-}[/latex] | 3.5 × 10−4 |
| [latex]\text{HNO}_2\;+\;\text{H}_2\text{O}\;{\rightleftharpoons}\;\text{H}_3\text{O}^{+}\;+\;\text{NO}_2^{\;\;-}[/latex] | 4.6 × 10−4 |
| [latex]\text{HCNO}\;+\;\text{H}_2\text{O}\;{\rightleftharpoons}\;\text{H}_3\text{O}^{+}\;+\;\text{NCO}^{-}[/latex] | 2 × 10−4 |
| [latex]\text{HCO}_2\text{H}\;+\;\text{H}_2\text{O}\;{\rightleftharpoons}\;\text{H}_3\text{O}^{+}\;+\;\text{HCO}_2^{\;\;-}[/latex] | 1.8 × 10−4 |
| [latex]\text{CH}_3\text{CO}_2\text{H}\;+\;\text{H}_2\text{O}\;{\rightleftharpoons}\;\text{H}_3\text{O}^{+}\;+\;\text{CH}_3\text{CO}_2^{\;\;-}[/latex] | 1.8 × 10−5 |
| [latex]\text{HCIO}\;+\;\text{H}_2\text{O}\;{\rightleftharpoons}\;\text{H}_3\text{O}^{+}\;+\;\text{CIO}^{-}[/latex] | 2.9 × 10−8 |
| [latex]\text{HBrO}\;+\;\text{H}_2\text{O}\;{\rightleftharpoons}\;\text{H}_3\text{O}^{+}\;+\;\text{BrO}^{-}[/latex] | 2.8 × 10−9 |
| [latex]\text{HCN}\;+\;\text{H}_2\text{O}\;{\rightleftharpoons}\;\text{H}_3\text{O}^{+}\;+\;\text{CN}^{-}[/latex] | 4.9 × 10−10 |
Table 17.7a gives the ionization constants for several weak acids; additional ionization constants can be found in Appendix I.
At equilibrium, a solution of a weak base in water is a mixture of the nonionized base, the conjugate acid of the weak base, and hydroxide ion with the nonionized base present in the greatest concentration. Thus, a weak base increases the hydroxide ion concentration in an aqueous solution (but not as much as the same amount of a strong base).
For example, a solution of the weak base trimethylamine, (CH3)3N, in water reacts according to the equation:
giving an equilibrium mixture with most of the base present as the nonionized amine. This equilibrium is analogous to that described for weak acids.
We can confirm by measuring the pH of an aqueous solution of a weak base of known concentration that only a fraction of the base reacts with water (Figure 17.7e). The remaining weak base is present as the unreacted form. The equilibrium constant for the ionization of a weak base, Kb, is called the ionization constant of the weak base, and is equal to the reaction quotient when the reaction is at equilibrium. For trimethylamine, at equilibrium:
The ionization constants of several weak bases are given in Table 17.7b and in Appendix J.
| Ionization Reaction | Kb at 25 °C |
|---|---|
| [latex](\text{CH}_3)_2\text{NH}\;+\;\text{H}_2\text{O}\;{\rightleftharpoons}\;(\text{CH}_3)_2\text{NH}_2^{\;\;+}\;+\;\text{OH}^{-}[/latex] | 5.9 × 10−4 |
| [latex]\text{CH}_3\text{NH}_2\;+\;\text{H}_2\text{O}\;{\rightleftharpoons}\;\text{CH}_3\text{NH}_3^{\;\;+}\;+\;\text{OH}^{-}[/latex] | 4.4 × 10−4 |
| [latex](\text{CH}_3)_3\text{N}\;+\;\text{H}_2\text{O}\;{\rightleftharpoons}\;(\text{CH}_3)_3\text{NH}^{+}\;+\;\text{OH}^{-}[/latex] | 6.3 × 10−5 |
| [latex]\text{NH}_3\;+\;\text{H}_2\text{O}\;{\rightleftharpoons}\;\text{NH}_4^{\;\;+}\;+\;\text{OH}^{-}[/latex] | 1.8 × 10−5 |
| [latex]\text{C}_6\text{H}_5\text{NH}_2\;+\;\text{H}_2\text{O}\;{\rightleftharpoons}\;\text{C}_6\text{N}_5\text{NH}_3^{\;\;+}\;+\;\text{OH}^{-}[/latex] | 4.3 × 10−10 |
Example 17.7c
Determination of Ka from Equilibrium Concentrations
Acetic acid is the principal ingredient in vinegar (Figure 17.7f); that’s why it tastes sour. At equilibrium, a solution contains [CH3CO2H] = 0.0787 M and [H3O+] = [CH3CO2–] = 0.00118M. What is the value of Ka for acetic acid?

Solution
We are asked to calculate an equilibrium constant from equilibrium concentrations. At equilibrium, the value of the equilibrium constant is equal to the reaction quotient for the reaction:
Exercise 17.7d
What is the equilibrium constant for the ionization of the HSO4– ion, the weak acid used in some household cleansers:
In one mixture of NaHSO4 and Na2SO4 at equilibrium, [H3O+] = 0.027M, [HSO4–] = 0.29M and [SO42-] = 0.13M.
Check Your Answer[3]
Example 17.7d
Determination of Kb from Equilibrium Concentrations
Caffeine, C8H10N4O2 is a weak base. What is the value of Kb for caffeine if a solution at equilibrium has [C8H10N4O2] = 0.050 M, [C8H10N4O2H+ ] = 5.0 × 10−3M, and [OH−] = 2.5 × 10−3M?
Solution
At equilibrium, the value of the equilibrium constant is equal to the reaction quotient for the reaction:
Exercise 17.7e
What is the equilibrium constant for the ionization of the HPO42- ion, a weak base:
In a solution containing a mixture of NaH2PO4 and Na2HPO4 at equilibrium, [OH−] = 1.3 × 10−6M, [H2PO4–] = 0.042M and [HPO42-] = 0.341M.
Check Your Answer[4]
Example 17.7e
Determination of Ka or Kb from pH
The pH of a 0.0516-M solution of nitrous acid, HNO2, is 2.34. What is its Ka?
Solution
We determine an equilibrium constant starting with the initial concentrations of HNO2, H3O+, and NO2– as well as one of the final concentrations, the concentration of hydronium ion at equilibrium. (Remember that pH is simply another way to express the concentration of hydronium ion.)
We can solve this problem with the following steps in which x is a change in concentration of a species in the reaction:
![A diagram is shown that includes six rust-red rectangles. The first rectangle is labeled, “p H,” and there is an arrow that points to the right to the second rectangle. The second rectangle is labeled, “[ H subscript 3 O superscript plus ].” There is an arrow that points right to the third rectangle. The third rectangle is labeled, “Calculate x subscript [ H subscript 3 O superscript plus ].” There is an arrow that points right to the fourth rectangle. The fourth rectangle is labeled, “Calculate x subscript [ H N O subscript 2 ] and x subscript [ N O subscript 2 superscript negative sign ].” There is an arrow that points down to the fifth rectangle. The fifth rectangle is labeled, “Calculate equilibrium concentrations.” There is an arrow that points down to the sixth rectangle. The sixth rectangle is labeled, “Calculate K subscript a.”](https://ecampusontario.pressbooks.pub/app/uploads/sites/2599/2022/05/CNX_Chem_14_03_steps_img.jpg)
We can summarize the various concentrations and changes as shown here (the concentration of water does not appear in the expression for the equilibrium constant, so we do not need to consider its concentration):
![This table has two main columns and four rows. The first row for the first column does not have a heading and then has the following in the first column: Initial concentration ( M ), Change ( M ), Equilibrium concentration ( M ). The second column has the header of, “H N O subscript 2 plus sign H subscript 2 O equilibrium arrow H subscript 3 O superscript positive sign plus sign N O subscript 2 superscript negative sign.” Under the second column is a subgroup of four columns and three rows. The first column has the following: 0.0516, negative x, [ H N O subscript 2 ] subscript i plus ( negative x ) equals 0.0516 plus sign ( negative x ). The second column is blank in the first row, positive sign, blank for the third row. The third column has the following: approximately 0, x, [ H subscript 3 O ] superscript positive sign plus sign x [ N O subscript 2 ] superscript negative sign plus sign x plus sign 0 plus sign x. The fourth column has the following: 0, x, 0.0046.](https://ecampusontario.pressbooks.pub/app/uploads/sites/2599/2022/05/CNX_Chem_14_03_ICETable1_img.jpg)
To get the various values in the ICE (Initial, Change, Equilibrium) table, we first calculate [H3O+, the equilibrium concentration of H3O+, from the pH:
The change in concentration of H3O+, [latex]x_{[\text{H}_3\text{O}^{+}]}[/latex], is the difference between the equilibrium concentration of H3O+, which we determined from the pH, and the initial concentration, [H3O+]. The initial concentration of H3O+is its concentration in pure water, which is so much less than the final concentration that we approximate it as zero (~0).
The change in concentration of NO2– is equal to the change in concentration of H3O+. For each 1 mol of H3O+ that forms, 1 mol of NO2– forms. The equilibrium concentration of HNO2 is equal to its initial concentration plus the change in its concentration.
Now we can fill in the ICE table with the concentrations at equilibrium, as shown here:
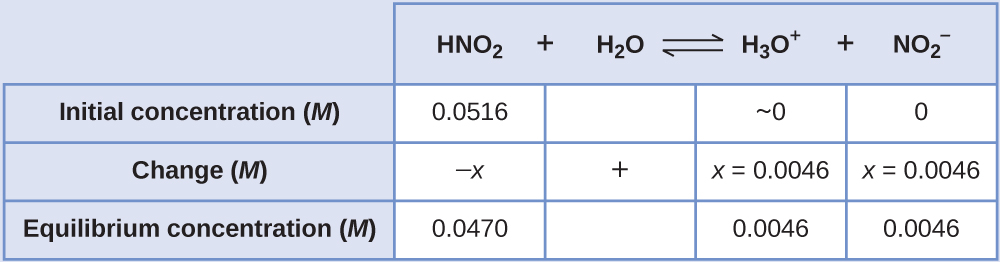
Finally, we calculate the value of the equilibrium constant using the data in the table:
Exercise 17.7f
Check Your Learning Exercise (Text Version)
The pH of a solution of household ammonia, a 0.950-M solution of NH3, is 11.612. What is Kb for NH3?
- 1.8 x 10-5
- 1.5 x 10-8
- 2.4 x 10-5
- 1.8 x 10-10
Check Your Answer[5]
Source: “Exercise 17.7f” is adapted from “Example 14.3-5” in General Chemistry 1 & 2, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson, licensed under CC BY 4.0.
Example 17.7f
Equilibrium Concentrations in a Solution of a Weak Acid
Formic acid, HCO2H, is the irritant that causes the body’s reaction to ant stings (Figure 17.7g).

What is the concentration of hydronium ion and the pH in a 0.534 M solution of formic acid?
Solution
- Determine x and equilibrium concentrations. The equilibrium expression is:
[latex]\text{HCO}_2\text{H}(aq)\;+\;\text{H}_2\text{O}(l)\;{\rightleftharpoons}\;\text{H}_3\text{O}^{+}(aq)\;+\;\text{HCO}_2^{\;\;-}(aq)[/latex]
The concentration of water does not appear in the expression for the equilibrium constant, so we do not need to consider its change in concentration when setting up the ICE table.
The table shows initial concentrations (concentrations before the acid ionizes), changes in concentration, and equilibrium concentrations follows (the data given in the problem appear in color):
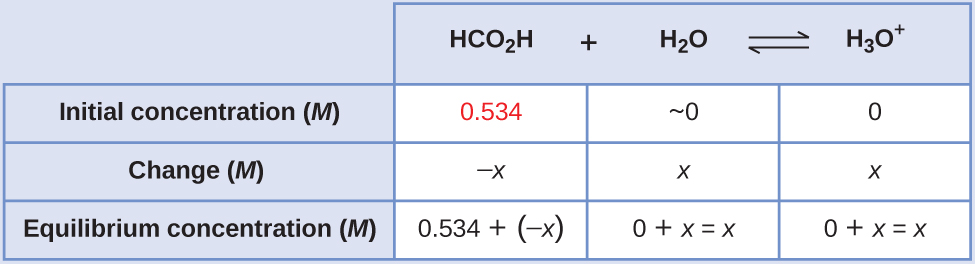
- Solve for x and the equilibrium concentrations. At equilibrium:
[latex]\begin{array}{r @{{}={}} l} K_{\text{a}} & = 1.8\;\times\;10^{-4}\\[0.5em] & = \frac{[\text{H}_3\text{O}^{+}][\text{HCO}_2^{\;\;-}]}{[\text{HCO}_2\text{H}]}\\[0.5em] & = \frac{(x)(x)}{0.534\;-\;x}\end{array}[/latex]
Now solve for x. Because the initial concentration of acid is reasonably large and Ka is very small, we assume that x << 0.534, which permits us to simplify the denominator term as (0.534 − x) = 0.534. This gives:
Solve for x as follows:
To check the assumption that x is small compared to 0.534, we calculate:
since x is less than 5% of the initial concentration (0.534 M), the assumption is valid.
We find the equilibrium concentration of hydronium ion in this formic acid solution from its initial concentration and the change in that concentration as indicated in the last line of the table:
The pH of the solution can be found by taking the negative log of the [latex][\text{H}_3\text{O}^{+}][/latex], so:
Exercise 17.7g
Only a small fraction of a weak acid ionizes in aqueous solution. What is the percent ionization of acetic acid in a 0.100-M solution of acetic acid, CH3CO2H?
(Hint: Determine [CH3CO2–] at equilibrium.) Recall that the percent ionization is the fraction of acetic acid that is ionized × 100, or
[latex]\frac{[\text{CH}_3\text{CO}_2^{\;\;-}]}{[\text{CH}_3\text{CO}_2\text{H}]_{\text{initial}}}\;\times\;100[/latex]
Check Your Answer[6]
The following example shows that the concentration of products produced by the ionization of a weak base can be determined by the same series of steps used with a weak acid.
Example 17.7g
Equilibrium Concentrations in a Solution of a Weak Base
Find the concentration of hydroxide ion in a 0.25 M solution of trimethylamine, a weak base:
Solution
This problem requires that we calculate an equilibrium concentration by determining concentration changes as the ionization of a base goes to equilibrium. The solution is approached in the same way as that for the ionization of formic acid in Example 17.7f. The reactants and products will be different and the numbers will be different, but the logic will be the same:
- Determine x and equilibrium concentrations. The table shows the changes and concentrations
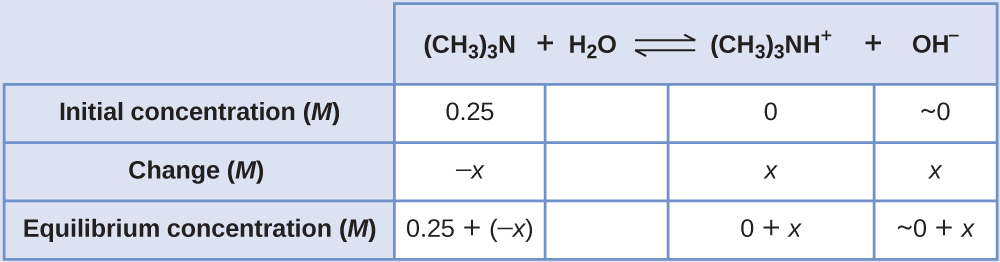
- Solve for x and the equilibrium concentrations. At equilibrium:
[latex]K_{\text{b}} = \frac{[(\text{CH}_3)_3\text{NH}^{+}][\text{OH}^{-}]}{[(\text{CH}_3)_3\text{N}]} = \frac{(x)(x)}{0.25\;-\;x} = 6.3\;\times\;10^{-5}[/latex]
If we assume that x is small relative to 0.25, then we can replace (0.25 − x) in the preceding equation with 0.25. Solving the simplified equation gives:
[latex]x = 4.0\;\times\;10^{-3}[/latex]This change is less than 5% of the initial concentration (0.25), so the assumption is justified.
Recall that, for this computation, x is equal to the equilibrium concentration of hydroxide ion in the solution (see earlier tabulation):
[latex]\begin{array}{r @{{}={}} l} [\text{OH}^{-}] & = {\sim}0\;+\;x\\[0.5em] & = x \\[0.5em] & = 4.0\;\times\;10^{-3}\;M\end{array}[/latex]Then calculate pOH as follows:
[latex]\text{pOH} = -\text{log}(4.0\;\times\;10^{-3}) = 2.40[/latex]Using the relation introduced in the previous section of this chapter:
[latex]\text{pH}\;+\;\text{pOH} = \text{p}K_{\text{w}} = 14.00[/latex]permits the computation of pH:
[latex]\text{pH} = 14.00\;-\;\text{pOH} = 14.00\;-\;2.40 = 11.60[/latex] - Check the work. A check of our arithmetic shows that Kb = 6.3 × 10−5.
Some weak acids and weak bases ionize to such an extent that the simplifying assumption that x is small relative to the initial concentration of the acid or base is inappropriate. As we solve for the equilibrium concentrations in such cases, we will see that we cannot neglect the change in the initial concentration of the acid or base, and we must solve the equilibrium equations by using the quadratic equation.
Example 17.7h
Equilibrium Concentrations in a Solution of a Weak Acid
Sodium bisulfate, NaHSO4, is used in some household cleansers because it contains the HSO4– ion, a weak acid. What is the pH of a 0.50-M solution of HSO4−?
Solution
We need to determine the equilibrium concentration of the hydronium ion that results from the ionization of HSO4− so that we can use [H3O+] to determine the pH. As in the previous examples, we can approach the solution by the following steps:

- Determine x and equilibrium concentrations. This table shows the changes and concentrations:
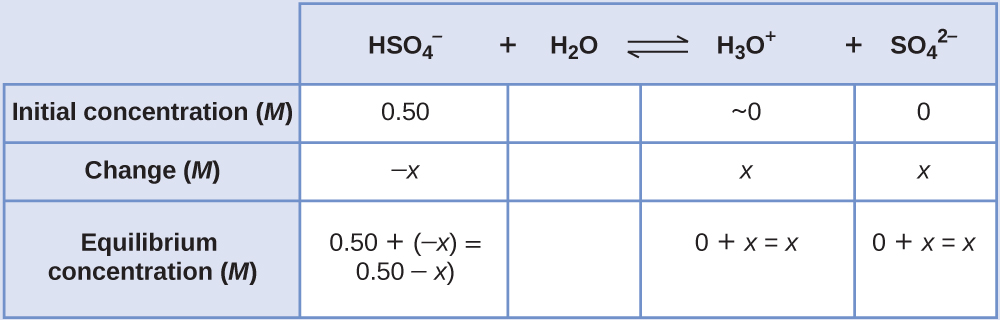
- Solve for x and the concentrations. As we begin solving for x, we will find this is more complicated than in previous examples. As we discuss these complications we should not lose track of the fact that it is still the purpose of this step to determine the value of x.
At equilibrium:
[latex]K_{\text{a}} = 1.2\;\times\;10^{-2} = \frac{[\text{H}_3\text{O}^{+}][\text{SO}_4^{\;\;2-}]}{[\text{HSO}_4^{\;\;-}]} = \frac{(x)(x)}{0.50\;-\;x}[/latex]If we assume that x is small and approximate (0.50 − x) as 0.50, we find:
[latex]x = 7.7\;\times\;10^{-2}[/latex]When we check the assumption, we calculate:
[latex]\frac{x}{[\text{HSO}_4^{\;\;-}]}_{\text{i}} \times(100) = \frac{7.7\;\times\;10^{-2}}{0.50}\times(100) = 15\%[/latex]The value of x is not less than 5% of 0.50, so the assumption is not valid. We need the quadratic formula to find x.
The equation:
[latex]K_{\text{a}} = 1.2\;\times\;10^{-2} = \frac{(x)(x)}{0.50\;-\;x}[/latex]gives
[latex]6.0\;\times\;10^{-3}\;-\;1.2\;\times\;10^{-2}x = x^{2}[/latex]or
[latex]x^{2}\;+\;1.2\;\times\;10^{-2}x\;-\;6.0\;\times\;10^{-3} = 0[/latex]This equation can be solved using the quadratic formula. For an equation of the form
[latex]ax^{2}\;+\;bx\;+\;c = 0[/latex],x is given by the equation:
[latex]x = \frac{-b\;{\pm}\;\sqrt{b^{2}\;-\;4\text{ac}}}{2a}[/latex]In this problem, a = 1, b = 1.2 × 10−3, and c = −6.0 × 10−3.
Solving for x gives a negative root (which cannot be correct since concentration cannot be negative) and a positive root:
[latex]x = 7.2\;\times\;10^{-2}[/latex]Now determine the hydronium ion concentration and the pH:
[latex][\text{H}_3\text{O}^{+}] = {\sim}0\;+\;x = 0\;+\;7.2\;\times\;10^{-2}\;M[/latex][latex]= 7.2\;\times\;10^{-2}\;M[/latex]The pH of this solution is:
[latex]\text{pH} = -\text{log}[\text{H}_3\text{O}^{+}] = -\text{log}\;7.2\;\times\;10^{-2} = 1.14[/latex]
Exercise 17.7h
Calculate the pH in a 0.010-M solution of caffeine, a weak base:
(Hint: It will be necessary to convert [OH−] to [H3O+] or pOH to pH toward the end of the calculation.)
Check Your Answer[7]
Key Equations
- [latex]K_{\text{a}} = \frac{[\text{H}_3\text{O}^{+}][\text{A}^{-}]}{[\text{HA}]}[/latex]
- [latex]K_{\text{b}} = \frac{[\text{HB}^{+}][\text{OH}^{-}]}{[\text{B}]}[/latex]
- [latex]K_{\text{a}}\;\times\;K_{\text{b}} = 1.0\;\times\;10^{-14} = K_{\text{w}}[/latex]
- [latex]\text{Percent ionization} = \frac{[\text{H}_3\text{O}^{+}]_{\text{eq}}}{[\text{HA}]_{0}}\;\times\;100[/latex]
Attribution & References
equilibrium constant for the ionization of a weak acid
ratio of the concentration of the ionized acid to the initial acid concentration, times 100
equilibrium constant for the ionization of a weak base

