2.1
Atomic and Molecular Structure
| Matter |
|
| Atom |
|
Atomic Structure
An atom is a central nucleus with orbiting electrons. The identity is determined by the composition of the nucleus and the arrangement of orbiting electrons. The arrangement within the atom is similar to that of the solar system and an atom has a nucleus as its center or sun, and the electrons revolve (orbit) around it like planets. The electrons remain stable in their orbit unless disturbed or moved. Orbiting electrons can be disturbed by x-rays.
Below is a diagram of a simplified atomic structure with labeled parts, including the nucleus comprised of protons and neutrons and surrounding orbital electrons.
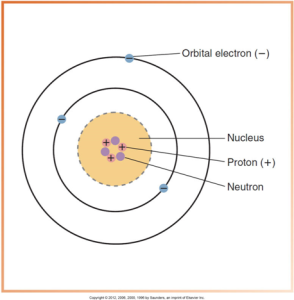
Nucleus
Nucleus is composed of protons and neutrons, and most of the atom is empty space. Protons have positive electrical charges and neutrons carry no electrical charge. The number of protons and neutrons in the nucleus of an atom determines its mass number or atomic weight where as the number of protons inside the nucleus equals the number of electrons outside the nucleus and determines the atomic number. Atoms are arranged in increasing atomic number on the periodic table of the elements.
Electrons
Electrons are tiny, negatively charged particles. These have very little mass, approximately 1/1800 as much as a proton or neutron. Electrons travel around the nucleus in well-defined paths known as orbits or shells. The shell located closest to the nucleus has the highest energy level.
Electrons are maintained in their orbits by electrostatic force between the positive nucleus and negative electrons. They are known as the binding energy of an electron. Binding energy is determined by the distance between the nucleus and the orbiting electron. The strongest binding energy is found closest to the nucleus in the K shell, and it is measured in electron volts or kilo electron volts.
Molecular Structure
Atoms are capable of combining with one another to form molecules. Molecules are two or more atoms joined by chemical bonds or the smallest amount of a substance that possesses its characteristic properties.
Molecules are formed in two ways: the transfer of electrons and the sharing of electrons between the outermost shells of atoms.
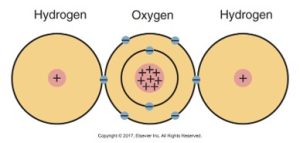
Below is a diagram of a water molecule (H2O) with two hydrogen atoms and one central oxygen atom connected by lines representing chemical bonds.
Ionization
Normally, most atoms are neutral. A neutral atom contains an equal number of protons and electrons. An atom with an incompletely filled outer shell attempts to capture an electron from an adjacent atom.
If the atom gains an electron, it has a negative charge or if the atom loses an electron, it has a positive charge. An atom that gains or loses an electron and becomes electrically unbalanced is called an ion.
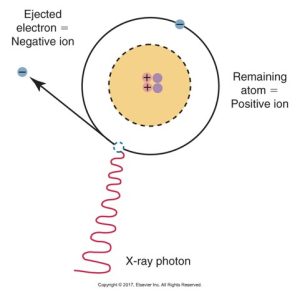
Ionization is the production of ions, or the process of converting an atom into ions. When an electron is removed from an atom in the ionization process, an ion pair results: the atom becomes the positive ion, and the ejected electron becomes the negative ion.
Below is a diagram of an atom undergoing ionization with an ejected electron forming a negative ion and the remaining atom as a positive ion caused by an incoming X-ray photon.
Radiation & Radioactivity
Radiation is the emission and propagation of energy through space or a substance in the form of waves or particles.
Radioactivity is the process by which certain unstable atoms or elements undergo spontaneous disintegration, or decay, in an effort to attain a more balanced nuclear state.
Ionizing Radiation
Radiation is capable of producing ions by removing or adding an electron to an atom and is classified into two groups: particulate radiation and electromagnetic radiation.
Particulate Radiation
Particulate radiation is tiny particles of matter that possess mass and travel in straight lines and at high speeds. There are four types: Electrons, Alpha Particles, Protons, and Neutrons.
1. Electrons: Beta particles are fast-moving electrons emitted from the nucleus of radioactive atoms. Cathode rays are streams of high-speed electrons that originate in an x-ray tube, which are electrons emitted by a manufactured device
2. Alpha Particles: Alpha particles are emitted from the nuclei of heavy metals. They exist as two protons and neutrons without electrons.
3. Protons: Accelerated hydrogen nuclei with a mass of 1 and charge of +1.
4. Neutrons: Accelerated particles with a mass of 1 and no electrical charge.
Electromagnetic Radiation
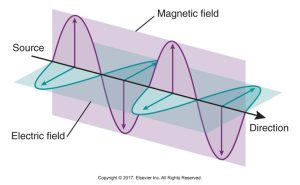
Electromagnetic radiation is the propagation of wavelike energy (without mass) through space or matter. Oscillating electric and magnetic fields are positioned at right angles to one another. They may be artificial or occur naturally and are arranged in the electromagnetic spectrum according to their energies.
Below is a diagram showing electromagnetic radiation with oscillating electric and magnetic fields at perpendicular angles to each other and the direction of propagation indicated by arrows.
An electromagnetic energy spectrum shows different wavelengths and frequencies. Each segment is labeled with a type of radiation technology or natural phenomena, ranging from radio waves to gamma-rays.
All electromagnetic radiations have common characteristics. They may be classified as ionizing or non-ionizing. Only high-energy radiations are capable of ionization and are believed to move through space as both a particle and a wave; both concepts must be considered.
Below is an infographic showcasing the electromagnetic spectrum, illustrated by a wavy line representing different wavelengths and frequencies.
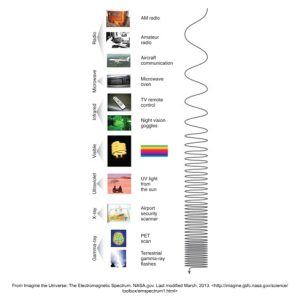
Particle Concept
The particle concept characterizes electromagnetic radiation in terms of discrete bundles of energy called photons or quanta. Photons are bundles of energy with no mass or weight. They travel as waves at the speed of light and move through space in a straight line.
Wave Concept
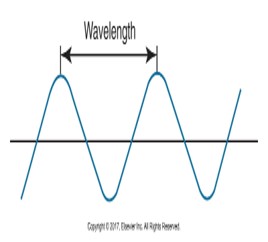
Wave concept characterizes electromagnetic radiations as waves of velocity (the speed of the wave), wavelength (the distance between the crest of one wave and the crest of the next), and frequency (the number of wavelengths that pass a certain point in a given length of time).
Below is a diagram of a sine wave representing a light wave or sound wave with the wavelength marked between two peaks.
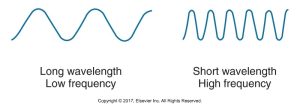
Frequency is the number of wavelengths that pass a given point in a certain amount of time. The shorter the wavelength, the higher the frequency, and vice versa.
Below is a diagram that shows the comparison of two sine waves: one with a long wavelength and low frequency and the other with a short wavelength and high frequency.
Media Attributions
- Iannucci & Howerton: Dental Radiography Principles and Techniques, 6th Edition, Chapter 2, CC BY-NC-ND

