Chapter 2: Enzyme-coupled Receptors
2.1 Introduction
The fundamentals of biochemical processes in the body rely on intricate and highly complex enzymatic signalling cascades. These signalling pathways may employ receptors situated at the cell surface (e.g. enzyme-coupled receptors, G-protein coupled receptors) or nuclear receptors. This unit will examine the structural and functional properties of various enzyme-coupled receptors.
2.1.1 What are enzyme-coupled receptors?
The term enzyme-coupled receptors can be examined as bringing together the two discrete components – receptors and enzymes. These proteins are receptors that also possess enzyme activity – the ability to catalyze a reaction. Receptors are transmembrane proteins, and they interact with a signal or ligand. This interaction occurs on the extracellular side of the cell and leads to a conformational change in the protein leading to enzymatic activity on the intracellular side of the cell. In this way, enzyme coupled receptors enable cellular signalling processes.
There are six major classes of enzyme-coupled receptors (Alberts, 2002).
I. Receptor tyrosine kinases (RTK)
II. Tyrosine-kinase-associated receptors
III. Receptor serine/threonine kinases
IV. Histidine-kinase-associated receptors
V. Receptor guanylyl cyclases
VI. Receptor-like tyrosine phosphatases
2.2 Chapter Specific Learning Outcomes
Upon successful completion of this chapter, you will be able to:
- Describe structural details of different enzyme-coupled receptors
- Compare target amino acids which are phosphorylated by different enzyme-couple receptors
- Describe processes by which inactive receptors are converted into active receptors
- Provide examples of different enzyme receptors
- Describe the series of phosphorylation events which take place during signalling modules (e.g. insulin signalling pathway, MAPK pathway)
- Describe different protein domains involved in protein-protein interactions participating in cell signalling
- Define Ras and compare its’ role to a molecular switch
- Differentiate between GAPs and GEFs and provide examples from the MAPK signalling pathway
- Distinguish between adaptor proteins and scaffold proteins in signalling pathways and identify an example
- Describe an overview of the JAK-STAT pathway
- Describe an overview of the TGFβ pathway
- Describe an overview of the two-component signalling system
- Give examples of different domains commonly involved in mediating protein interactions in signalling modules
2.3 Overview of RTK and their protein partners
2.3.1 What is the generic structural architecture of a RTK?
There are 58 RTKs known to exist within the human genome and they respond to different extracellular signals (Robinson et al., 2000). However, all RTKs possess common structural domains: an extracellular ligand binding domain, a transmembrane helix, a juxtamembrane region, and a tyrosine kinase (TK) domain.
RTKs are integral proteins that extend through the plasma membrane (via the transmembrane domain which is a single helix). The N-terminal region of the receptor is located in the extracellular region, while the C-terminal portion is situated in the cytoplasm.
The N-terminal region of the RTK contains the extracellular ligand binding site, which is the location of where an external signal will engage with the protein. This extracellular region is highly variable between different RTKs, and may include Cys-rich regions, Leu-rich segments, or immunoglobulin-like motifs. This helps maintain diversity for recognition of different external biochemical signals (Lemmon & Schlessinger, 2010).
The C-terminal region exists within the interior of the cell (the cytosol) and includes the conserved tyrosine kinase domain and juxtamembrane region. This kinase domain carries out the phosphorylation activity of the RTK whereas the juxtamembrane is highly flexible and important for regulatory roles, often leading to auto-inhibition via contacts with kinase domain.
2.3.2 Which amino acids on protein substrates are phosphorylated by RTKs?
a. Ser
b. Thr
c. Tyr
d. Option a and b
RTKs phosphorylate Tyr residues on protein substrates.
2.3.3 Which phosphate group is transferred during the phosphorylation step by RTKs?
a. α
b. β
c. γ
d. α and β
e. β and γ
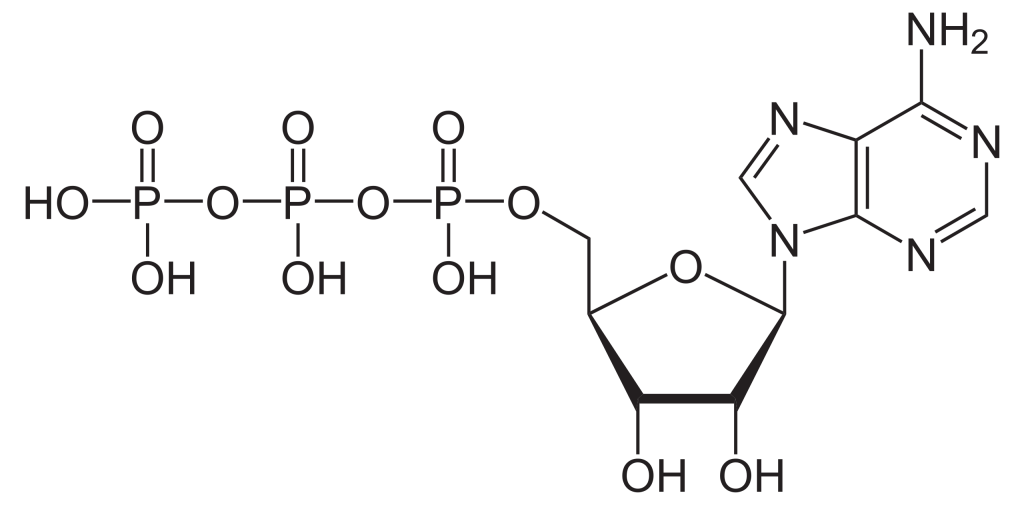
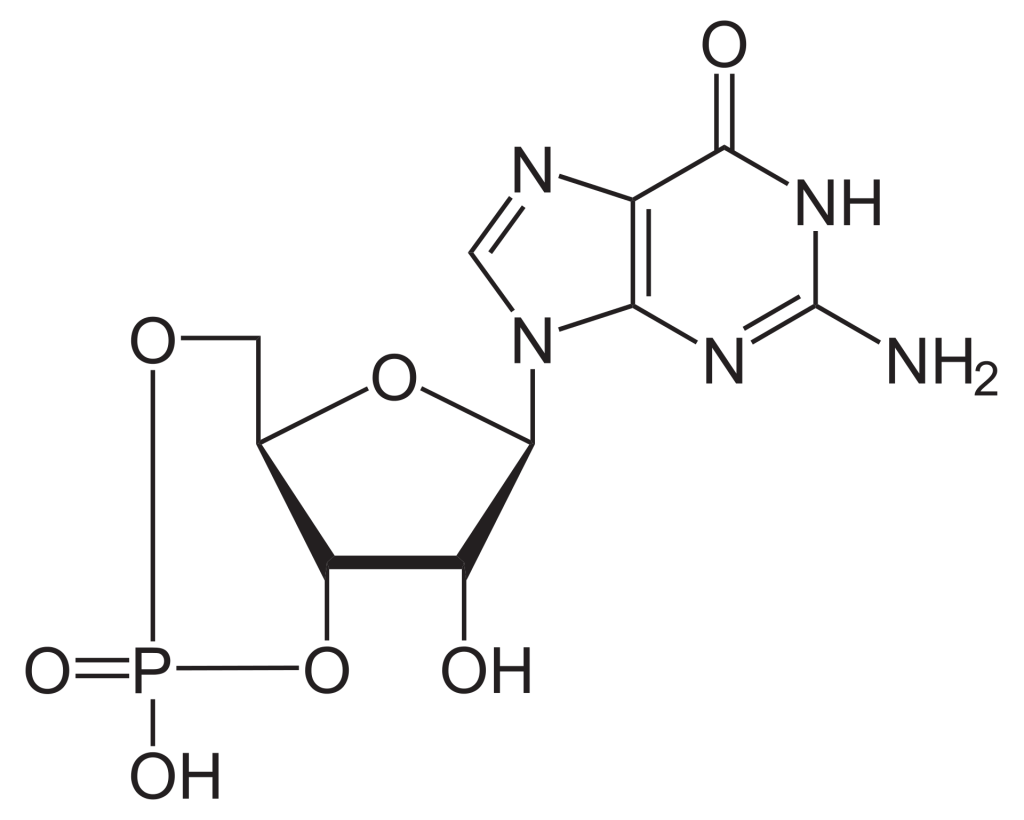
To build the nucleotide, ATP, three essential components are required: a five carbon ribose sugar, a nitrogenous base (adenine) and three phosphate groups which are labelled, α, β and γ (Figure 2.1). The α phosphate is positioned closest to the sugar group while the γ phosphate is furthest away. RTKs transfer a phosphate group from ATP adding a phosphate group to Tyr on protein substrates. The γ phosphate group is transferred during the phosphorylation step to a protein substrate. This is a highly exergonic reaction.
2.3.4 Are RTKs monomeric or dimeric?
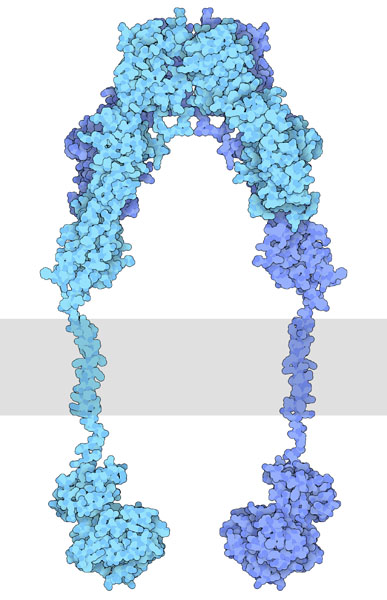
In the ‘inactive state’ RTKs generally exist as a single protein which is referred to as a monomer. However, the ‘active’ form requires two RTK proteins to come in close proximity which is called a dimer (Figure 2.2). However, there are exceptions. For example, the insulin receptor (INSR or IR) also exists as a dimer in the inactive form (Figure 2.3) due to a cysteine disulfide bridge linkage between the monomeric species.
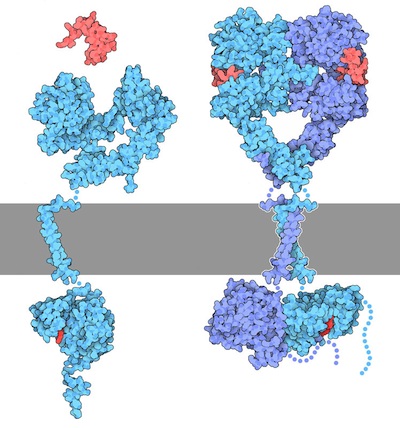
2.3.5 Describe the mechanism by which inactive RTKs are converted to active RTKs.
For this mechanism, we will consider RTKs that are initially in an inactive monomeric form. When an appropriate ligand binds to the receptor at the extracellular ligand binding domain, this results in a conformational change which converts the inactive RTK to the active form and facilitates bringing two monomer RTKs together. These two monomers are positioned in close proximity to each other, leading to the formation of a dimer.
Recall, the TK domain is located in the cytosolic portion of the receptor. Once the dimer is formed, the TK domains are positioned to carry out ‘trans-autophosphorylation.’ This is where a TK domain on one monomer phosphorylates selective Tyr residues located on the opposite monomer. This “trans-phosphorylation” converts the kinase domain into an active kinase domain, and the inactive RTK is converted to an active RTK.
2.3.6 Do RTKs possess allosteric enzymatic activity?
Yes, ligand binding (e.g. epidermal growth factor, EGF) to the ligand binding domain of the receptor (e.g. epidermal growth factor receptor – EGF receptor) promotes conformational changes, eventually activating the RTK enzymatic activity (Figure 2.2). In this way, signals external to the cell are transmitted to the cellular interior, without the molecule/hormone crossing the membrane.
2.3.7 A mutated RTK is missing the ligand binding domain but continues to produce a cellular response. Provide a potential negative consequence of this mutation.
In this mutated RTK, the transmembrane and cytoplasmic domain are assumed to be functionally and structurally intact. However, as the ligand binding domain absent, there is no regulatory response and the RTK is no longer sensitive to its cognate ligand. In this case, the receptor will be in a perpetual ‘on’ state or constitutively active state. A negative consequence is a receptor which is ligand-independent can lead to hyper-phosphorylation and hyper-activation of downstream RTK pathways. In fact, 30% of human cancers have been demonstrated to have hyperactivated or overexpressed RTKs (Ségaliny et al., 2015).
2.3.8 If EGFR recruits SHP2, predict the impact that SHP2 would have.
a. Inhibit EGFR activity.
b. Activate EGFR activity.
As previously discussed, SHP2 is a phosphatase. In close proximity to EGFR, it would likely remove the phosphate group from EGFR (Lemmon & Schlessinger, 2010) which would reduce the activity of this RTK.
2.3.9 Describe a key purpose of phospho-Tyr residues in signalling pathways.
Following trans-autophosphorylation, RTKs possess phospho-Tyr residues. These residues function as sites to enable the binding and recruitment of specific signalling proteins. The regions on the RTK which contain the phospho-Tyr residues are also referred to as docking sites or binding sites. Signalling proteins which bind to this region possess phospho-Tyr-binding domains.
2.3.10 Where is the location of the ligand-binding domain in INSR?
a. Extracellular region
b. Transmembrane portion of the receptor
c. Intracellular region
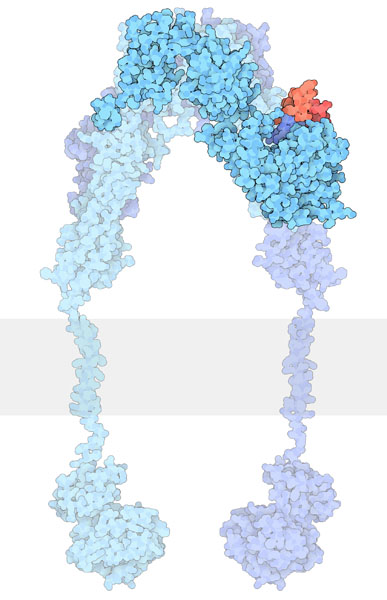
Some RTKs such as INSR exist as dimers in the inactive form. Similar to other RTKs, such as EGFR, the ligand binding domain is located in the extracellular domain (Figure 2.4).
2.3.11 Which amino acid (or amino acids) form disulfide bridges under oxidizing environments?
a. Ser
b. Met
c. Cys
d. Cys and Met
e. Cys, Met, Ser
INSR contains disulfide bonds which are formed from two Cys residues.
2.3.12 How is INSR converted from an inactive dimer to an active dimer?
Similar to other RTKs, the ligand binds to the receptor and this interaction results in a conformational change which activates the tyrosine kinase domain. Each monomer (in blue) can phosphorylate the opposing monomeric unit (Figure 2.4). The active tyrosine domain carries out the ‘autophosphorylation’ step, phosphorylating the other unit at tyrosine residues.
2.3.13 Predict what would occur if INSR was treated with a reducing agent (e.g. 2 mM Dithiothreitol (DTT)).
a. INSR activity would be enhanced.
b. INSR activity would decrease.
c. No effect on INSR activity.
DTT is a reducing agent, which would target the disulfide bonds, catalyzing their reduction. Treatment with DTT would be predicted to decrease receptor activity (Pike et al., 1986).
2.3.14 During the “autophosphorylation” step, which residues are phosphorylated on INSR?
a. Cys
b. Met
c. Tyr
INSR is a RTK, where Tyr residues are phosphorylated.
2.3.15 As INSR participates in autophosphorylation, does it also phosphorylate other targets?
INSR (in the active state) phosphorylates other cellular targets, including a common target Insulin Receptor Substrate (IRS).
2.3.16 Do signalling proteins which bind phospho-Tyr possess unique protein domains?
Proteins associated with RTK pathways often possess SH2 (Src homology region) domains or PTB (phosphotyrosine-binding) domains for phosphotyrosine binding. To illustrate an example, IRS-1 binds the phosphorylated receptor using its PTB domain. Another case involves Grb2 which binds phophotyrosine containing motifs through an SH2 domain.
2.3.17 Describe the structure of a SH2 domain.
The SH2 domain is a protein domain that serves to bind phospho-tyrosine residues. The SH2 domain has two sub-pockets which are formed by two alpha helices that are separated by a central β sheet (comprised of three β strands). The phospho-tyrosine residue inserts into one sub-pocket (called the pY pocket) on one side the central β sheet, and residues C-terminal to the phospho-tyrosine insert into the other sub-pocket (called the pY + 3 pocket) on the other side of the β sheet (Bajusz et al., 2023). These flanking residues confer specificity via key amino acid side chains. Therefore, different SH2 domains will recognize phospho-tyrosine containing proteins within the context of the surrounding amino acids. This mechanism of action of a SH2 domain is often compared to a plug and a two-pronged socket (Waksman et al., 1993).
2.3.18 Explain how the binding of insulin to its receptor leads to a series of phosphorylation events.
Insulin (ligand) binds to the cognate receptor, INSR, located at the cell surface of specific tissues (Figure 2.5). This results in a conformational change and subsequent auto-phosphorylation of specific Tyr residues on INSR. The activated INSR phosphorylates targets including the adaptor protein insulin receptor substrates (e.g. IRS-1). IRS-1 (in its phosphorylated state) serves as a docking site for a kinase, Phosphatidylinositide 3-kinase (PI3K). The enzyme PI3K catalyzes the phosphorylation of Phosphatidylinositol 4,5-bisphosphate (PIP2) to Phosphatidylinositol-3,4,5-triphosphate (PIP3). PIP3 activates another kinase, PDK1 (PIP3-dependent protein kinase). In the active form, PDK1, phosphorylates other kinases including Protein kinase B (PKB, also known as Akt1). Similarly, mTORC2 also phosphorylates and activates PKB. Continuing the series of phosphorylation events, PKB, phosphorylates targets including glycogen synthase kinase 3 (GSK3). In this case, phosphorylation inactivates the enzyme GSK3. In the phosphorylated form, GSK3, is unable to inactivate glycogen synthase (GS). GS is an enzyme which participates in converting glucose into glycogen.
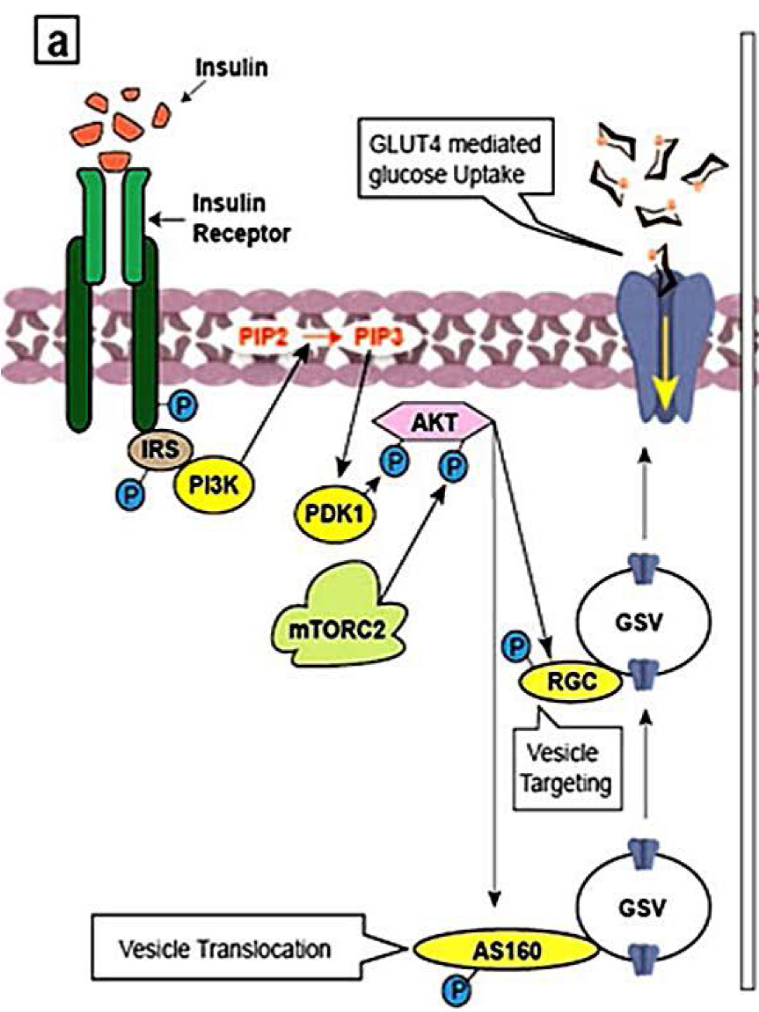
2.3.19 Describe the protein complex mTOR complex 2 (mTORC2)
Here the term mTORC2 will be parsed further. TOR is a protein kinase and the mammalian form is referred to as mTOR or mammalian target of rapamycin. Together two different protein complexes mTORC1 and mTORC2 form mTOR. As a Ser/Thr protein kinase, mTOR phosphorylates Ser or Thr (whereas the original phosphorylation events were phospho-Tyr driven). The protein complex, mTORC2 is classically activated by variety of elements ranging from amino acids and growth factors such as insulin (Tato et al., 2011; Yoon, 2017) (Figure 2.5).
2.3.20 How does Glucose transporter type 4 (GLUT4) play a role in glucose uptake?
PKB also recruits and activates insulin-sensitive GLUT4-containing vesicles to promote glucose transport. PKB phosphorylates the GTPases, RAB GAP AS160 and RAL-GAP complex (RGC) (Figure 2.5) (Sayem et al., 2018). These are GTPases that promote the transport of GLUT4 vesicles to the plasma membrane. GLUT4 vesicles contain glucose transporter (GLUT4) which are incorporated in the plasma membrane. These proteins facilitate the transport of glucose from the blood into the cell and are known for making cells “permeable” to glucose.
2.3.21 What is Ras?
Ras refers to a family of proteins which are small monomeric GTPases. Ras consists of isoforms (e.g. H-ras, K-ras, N-ras). The GTP binding protein Ras was identified in Rat sarcoma, hence forming the basis for the name.
2.3.22 The small protein, Ras, is often compared to a light switch. Why does this analogy suit the role of Ras?
The light switch (Figure 2.6) toggles between the ‘ON’ and ‘OFF’ options, where light appears in the ‘ON’ state and light is turned off in the ‘OFF’ state.
Similarly, when Ras is bound to the nucleotide guanosine triphosphate (GTP) it is in the active state.
Ras + GTP → active or ‘ON’
Whereas, when Ras is bound to the nucleotide guanosine diphosphate (GDP), Ras is in the inactive state.
Ras + GDP → inactive or ‘OFF’
Therefore, Ras is analogous to a switch, existing in two forms (active and inactive) (Figure 2.6).

2.3.23 Differentiate between GTP and GDP structurally.
GTP is a nucleotide composed of three key components: five carbon sugar (ribose), nitrogenous base (guanine) and three phosphate groups. GDP is a nucleotide formed from the same components except that it possesses two phosphate groups.
2.3.24 How is Ras able to switch from being bound from GDP to GTP?
Guanosine nucleotide-exchange factors (GEFs) are typically proteins which catalyze the exchange of GDP to GTP. When Ras is bound to GDP, GEF catalyzes the switch of GDP to GTP.
2.3.25 How is Ras able to switch from being bound from GTP to GDP?
GTPase-activating proteins (GAPs) support the hydrolysis of GTP to GDP. When Ras is bound to GTP, GAPs catalyze the switch from GTP to GDP. Ras is now bound to GDP.
2.3.26 RTKs are linked to Mitogen-activated protein kinase (MAPK) pathways. Define MAPK.
Mitogen-activated protein kinases (MAPKs) or MAP Kinases are an ubiquitous group of enzymes present in eukaryotes (Figure 2.7). In mammals the following representative groupings (i-iii) are members of the MAPK family (Morrison, 2012).
i. c-Jun NH2-terminal kinases (JNKs)
ii. Extracellular signal-regulated kinases (ERKs)
iii. p38 mitogen-activated protein kinases (p38s)
2.3.27 Which of the following amino acid or amino acid(s) are phosphorylated by members of the MAPK family?
a. Ser
b. Thr
c. Tyr
d. Option a or b
e. All of the above are true
Members of the MAPK family phosphorylate Ser or Thr residues.
2.3.28 Define Mitogen-activated protein kinase kinase (MAPKK) and Mitogen-activated protein kinase kinase kinase (MAPKKK)
Members of the MAPKK and MAPKKK family phosphorylate both Ser or Thr.
A representative MAPKKK, c-Raf (Figure 2.7) phosphorylates specific Ser residues on MEK. Members of the MAPKK family phosphorylate Ser, Thr residues of the MAPK family.
2.3.29 Summarize the Nomenclature with MAPK Signalling pathway:
Within the literature, there are various names used to describe the following kinases. Match the following kinase with alternative names used to describe them (Table 1).
| Kinase | Alternative Name |
| MAPK | MAP kinase |
| MAPKK | MAP2K or MAP kinase kinase |
| MAPKKK | MKKK or M3K or MAP3K or MAP kinase kinase kinase |
| MAPKKKK | MAP4K or MAP kinase kinase kinase kinase |
2.3.30 Why is the MAPK signalling pathway also referred to as a MAPK cascade?
Within the MAPK signalling pathway, there are a series of phosphorylation events where MAPKKK initially phosphorylates MAPKK, which phosphorylates MAPK (Figure 2.7). A relay or ‘cascade’ of phosphorylation reactions takes place (Meister et al., 2013). MAPK will then proceed to phosphorylate additional proteins such as transcription factors.
2.3.31 Why is MEK referred to as a dual-specificity protein kinase?
MEK phosphorylates both Ser/Thr or Tyr residues on the protein substrate (Table 2). For example, MEK phosphorylates specific Thr and Tyr residues on Erk (Zheng & Guan, 1993).
2.3.32 Match the roles for various components in the MAPK pathway:
| Component | Role |
| Ras | Monomeric G protein |
| Raf | Ser/Thr Kinase |
| MEK | Dual-specificity protein kinase |
| Erk | Ser/Thr Kinase |
2.3.33 What are some biological outcomes from the MAPK pathway?
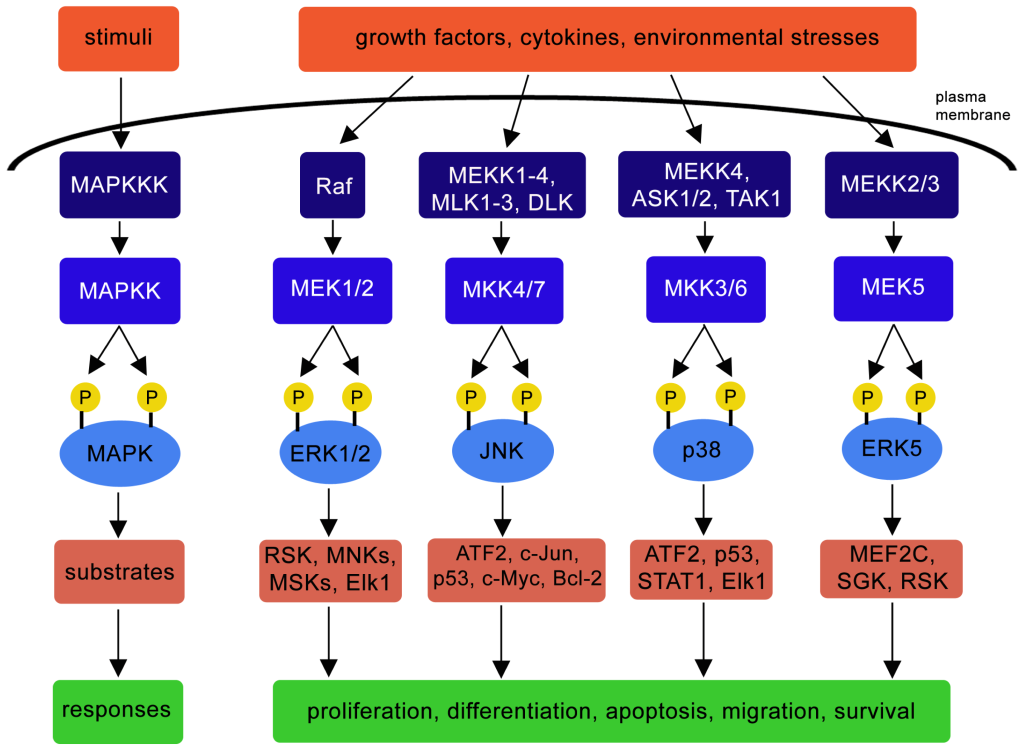
MAPK pathway is involved in a spectrum of biological outcomes including promoting cell proliferation, cell differentiation, apoptosis, migration, cell survival depending on the kinase and corresponding substrates (Figure 2.8) (Osaki & Gama, 2013).
For example, a signal (e.g. cytokine) may activate the MAPKKK, apoptosis signal-regulating kinase 1 (ASK1) (Ichijo et al., 1997) (Figure 2.8). A series of phosphorylation events ensue, such as the phosphorylation of MKK3 and MKK6 (both MAPKKs). Both phosphorylated MAPKKs can activate p38 (a MAPK) through phosphorylation events. Activated p38 targets a number of substrates such as activating transcription factor 2 (ATF2), p53, STAT1, Elk1 resulting in different cellular responses (Figure 2.8). Targeted Ser phosphorylation by p38 of the tumor suppressor p53 promotes one cellular response – apoptosis (Bulavin et al., 1999).
2.3.34 Provide a link between IRS and the MAPK cascade?
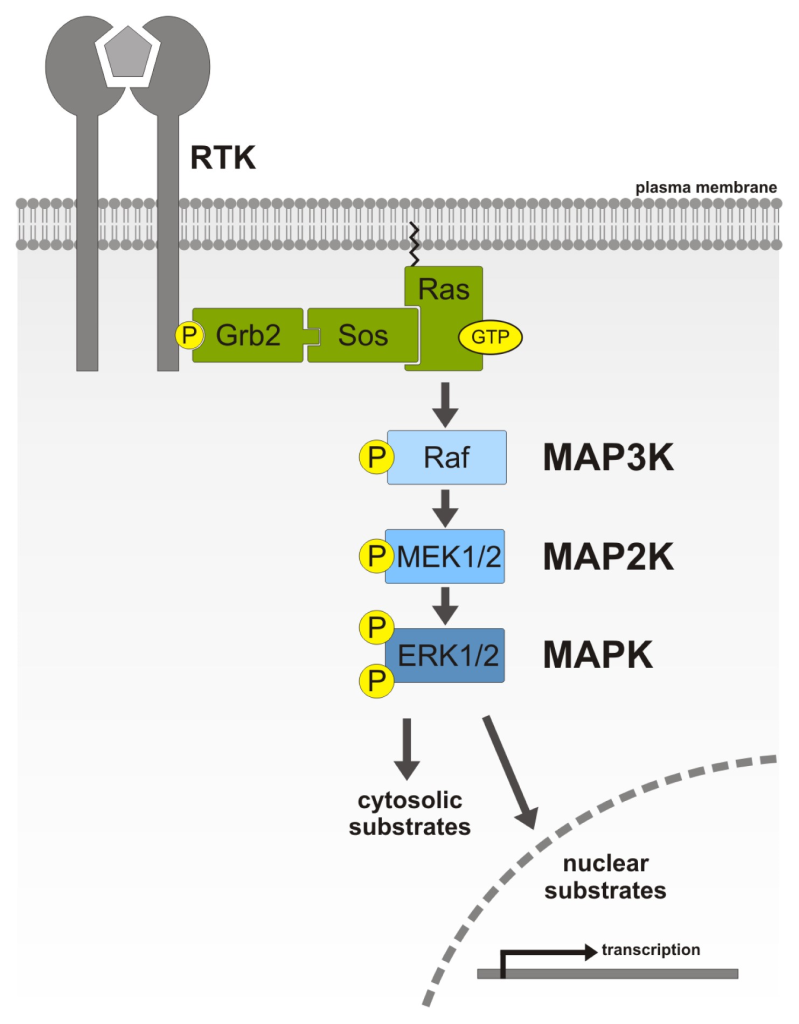
IRS in the phosphorylated state recruits Grb2 (Growth factor receptor-bound protein 2). Grb2 binds to IRS-1 via the Grb2 SH2 domain. Following this, Grb2 recruits the protein Sos. Sos is a GEF, and promotes the switch between the GDP bound and GTP bound state in G-proteins. One of the key proteins that Sos associates with is Ras, and Ras is converted to the GTP bound state.
Recall, Ras-GTP is now in the active state and Ras can activate MAPKKK (Raf-1) (Figure 2.7). This leads to a series of phosphorylation steps part of the MAPK cascade.
2.3.35 How is the interaction between Ras and the plasma membrane mediated?
This interaction is mediated by an ‘anchor’ composed of:
a. Polypeptide chain
b. Hydrophobic lipid group (e.g. farnesyl group)
c. Ubiquitin tag
d. Hydrophilic sugar group
As part of the MAPK pathway, Ras is linked to the plasma membrane and is situated on the cytosolic face via a lipid anchor called a farnesyl group. The anchor is shown by the red structure embedded as part of the plasma membrane (gray) (Figure 2.9) located at the C-terminus (Zhou et al., 2018).
2.3.36 During the MAPK pathway, GDP is exchanged for GTP. Which protein is a GEF?
a. Sos
b. Ras
c. INSR
d. Grb2
Sos is an example of a guanine nucleotide exchange factor (GEF).
2.3.37 Define the role of adaptor proteins in cell signalling. State examples of an adaptor protein in the insulin signalling pathway.
In signalling pathways, adaptor proteins help to mediate interactions between other proteins. While adaptor proteins contain different protein domains (e.g. SH2 domains), catalytic activity is usually not ascribed to adaptor proteins. Grb2 is an example of an adaptor protein which participates in multiple signalling pathways, including the insulin signalling pathway. Grb2 contains an SH2 domain and two SH3 domains. The SH2 domain serves to bind an activated phosphotyrosine molecule, and SH3 domains recognize proline-rich sequences. As an adaptor protein, Grb2 serves as a link between the Sos protein (via SH3 domains) and the RTK (via the SH2 domain).
2.3.38 Erk can phosphorylate Sos, disrupting the Sos-Grb2 interactions (Gomperts et al., 2009). How will this affect the MAK pathway?
a. Halting the MAPK pathway
b. Activation of the MAPK activity
c. No effect of the pathway
Sos is a cytosolic protein. Grb2 binds to the proline-rich domains of Sos through the two SH3 domains (Buday et al., 1994). Following these interactions, Sos localizes towards the plasma membrane and is positioned near (the lipid-bound) Ras thereby promoting nucleotide exchange. However, in some situations, phosphorylation of Sos will disrupt the Sos-Grb2 complex, causing dissociation of these proteins, ultimately negatively regulating the MAPK pathway (S. Corbalan-Garcia S.-S. Yang & Bar-Sagi, 1996).
2.3.39 Describe a role of scaffold proteins, such as Kinase suppressor of Ras (KSR).
To highlight the role of scaffold proteins, consider the role of scaffolding when constructing large buildings. The scaffolding material provides support during the assembly process. In an analogous manner, KSR is a scaffold protein, enabling a surface for various kinases to assemble (e.g. Raf, MEK, MAPK) and position in close proximity to each other (Morrison, 2001).
2.4 Tyrosine-kinase associated receptors in signalling pathways
2.4.1 Compare and contrast between tyrosine-kinase associated receptors and RTKs?
Both classes of receptors are capable of carrying out tyrosine phosphorylation and have intracellular, extracellular, and transmembrane domains. However, RTKs directly harbour kinase domains in the cytosolic regions, whereas tyrosine-kinase associated receptors will associate with an independent kinase protein. The enzymatic catalytic activity is separate from the C-terminal domain of the receptor in tyrosine-kinase associated receptors.
2.4.2 How are kinase domains connected with tyrosine-kinase associated receptors?
a. Covalently
b. Non-covalently
The tyrosine-kinase domains are not directly part of the primary sequence of the receptor, and are separate entities. For instance, cytokine receptors do not have endogenous tyrosine kinase domains. Rather the tyrosine kinase activity is associated noncovalently with Janus kinases (JAKs).
2.4.3 What are cytokines?
Cytokines are the signalling molecules activating corresponding cytokine receptors. They are proteins that are often involved in promoting cell growth, hematopoiesis, and immune responses. There are blurred divisions between cytokines and hormones. For example, a growth hormone is often also considered a cytokine.
2.4.4 What triggers cytokine receptor dimerization?
Cytokine receptors typically exist as one unit or as monomers. Cytokine binding to the receptor causes a conformational change that enables formation of homodimers (if the receptors are identical). If two or more different types of receptors dimerize this is referred to a receptor heterodimerization.
2.4.5 How do cytokine receptors convert from an inactive to an active dimeric state?
The mechanism for activation of cytokine receptors is similar to RTKs, in the manner that ligand binding results in a conformational change, bringing the monomers in close vicinity to each other. The intracellular tyrosine kinases that are associated with each receptor will be positioned in an orientation that enables them to phosphorylate the opposing kinase.
For example, cytokine receptor dimerization positions JAKs, on the receptor and they carry out trans-autophosphorylation, where JAKs phosphorylate a tyrosine on the opposite receptor. This phosphorylation activates JAK which proceeds to phosphorylate multiple sites on the C-terminal tail of each receptor.
2.4.6 What is the role of JAK phosphorylation events in the JAK-STAT pathway?
Following phosphorylation of the cytokine receptor C-terminal tails, the phosphotyrosine serves as a binding site for different proteins including the signal transducer and activator of transcription (STAT) proteins. STAT proteins are transcription factors that contain several domains including SH2 domains. The SH2 domains serve two functions. Initially, these domains recognize and bind to the phosphotyrosine sites on the cytoplasmic C-terminal tails of the cytokine receptor. Following docking of the STAT proteins on the receptor, JAK will subsequently phosphorylate the C-terminal tail of STAT proteins (Figure 2.10). These phosphorylation events result in a conformational change, where the STAT proteins will separate from the cytokine receptor. As a result, the phoshphotyrosine of one STAT monomer will bind to the SH2 domain of another STAT protein, and vice versa, creating a homodimer. The STAT homodimer enters the nucleus and binds to regulatory DNA sequences enabling for transcription of targeted genes (Erdogan et al., 2022).
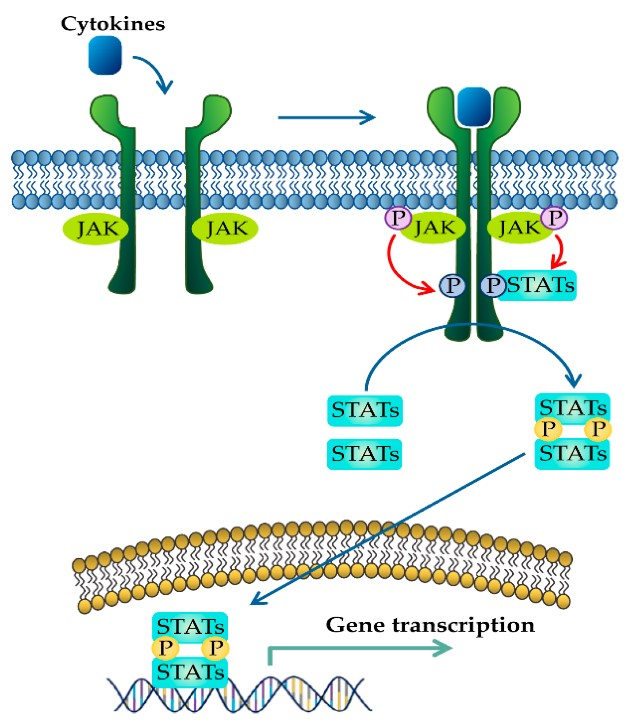
2.4.7 In some cases mutations in SH2 domain-containing proteins can lead to protein hyperactivation or loss-of-function, resulting in a range of disease states including cancers. Explain how this can occur.
SH2 domains bind phosphotyrosine containing motifs. Specific mutations in the SH2 domain can lead to distortion of the secondary structure of the protein. Some of these distortions can negatively affect the protein structure which leads to loss-of-function. For example, a mutation K591E in the STAT3 SH2 domain results in reduced STAT3 activity since the positively charged lysine chain is replaced by a negatively charged glutamic acid (de Araujo, Orlova, et al., 2019). Since this residue side chain directly coordinates the negatively charged phospho-tyrosine, it leads to electrostatic repulsion and reduced-activity. In a similar manner, a STAT5 N642H mutation results in gain-of-function activity of the protein and this mutant is found in multiple blood cancers (de Araujo, Erdogan, et al., 2019; de Araujo et al., 2020). This mutation directly increases the affinity of the SH2 domain for phosphotyrosine via coordinating the phosphate group, which ultimately leads to tighter interactions with the cytokine receptor as well as increased lifetime of the STAT dimer and prolonging transcriptional activity.
2.4.8 How are STAT proteins transported into the nucleus?
a. STAT proteins diffuse through the nuclear pore complex
b. STAT proteins require the aid of importins
c. STATs are unable to enter the nucleus
The nucleus possesses nuclear pore complexes, which are intercalated within the nuclear membrane enabling for diffusion of small molecules (less than 60 kDa). STATs require the aid of nuclear transport receptors – importins to mediate entry in the nucleus (Figure 2.11) (Reich, 2013).
2.5 Serine-threonine kinase receptors
In contrast to the RTKs, Ser/Thr kinase receptors (sometimes referred to as RSTK) are members of the enzyme-coupled receptor class that expectantly phosphorylate Ser and Thr residues on target proteins.
2.5.1 How do serine/threonine kinases operate in signalling pathways?
The prototypical example of RSTKs is the transforming growth factor β (TGFβ) receptors which can exist in both a Type I and Type II form. Both types have single membrane spanning domains as well as intracellular and extracellular domains. Ligands bind to the extracellular domains of Type II homodimer receptors followed by heterodimerization with Type I receptors to form a heterotetrameric complex. The intracellular ser/thr kinase domain carries out transphosphorylation. Additional modifications or variations in the C-terminal portion enable for additional layers of regulation.
2.5.2 What is the domain difference between type I and type II TGFβ receptors?
While both receptors possess Ser/Thr kinase domains, Type I possess an additional ‘GS domain’ (Gomperts et al., 2009).This domain is a Gly-Ser rich domain which can also be phosphorylated. Type II receptors possess a Cys rich extracellular domain.
2.5.3 Describe the interactions between type I and type II receptors in the TGFβ signalling pathway.
TGFβRII exists as a homodimer and the ligand (TGFβ) binds to the receptor (TGFβRII) resulting in a conformational change. TGFβRII binds to TGFβRI which enables serine phosphorylation. Specifically, TGFβII phosphorylates Ser rich region of the GS domain receptor of TGFβI (Tzavlaki & Moustakas, 2020). A chaperone protein (FK506 binding protein of 12 kilodalton – FKBP12) is also associated with TGFβRI. Ser phosphorylation-driven conformational changes and concurrent release of FKBP12 lead to activation of TGFβRI. At this stage, an active tetrameric receptor complex is assembled composed of two type II TGFβ receptors and two type II TGFβ receptors (Figure 2.12). Ligand binding by molecules such as TGF-β, activin, nodal, can lead to TGFβ association with Smad2 (Suppressor of Mothers against Decapentaplegic – Smad) and Smad3, while ligands such as bone morphogenetic protein (BMP) can recruit Smad1, 5, 8, or 9. In either case, Smad binding is also mediated by a membrane associated protein called SARA and the Smad protein will be phosphorylated by TGF-β. Phosphorylation of Smads results in dissociation of the phospho-Smad and SARA. The phospho-Smad forms a complex with another protein – Smad4 (Figure 2.12). Together, the phosphorylated Smad-Smad4 complex enters the nucleus to regulate gene expression.
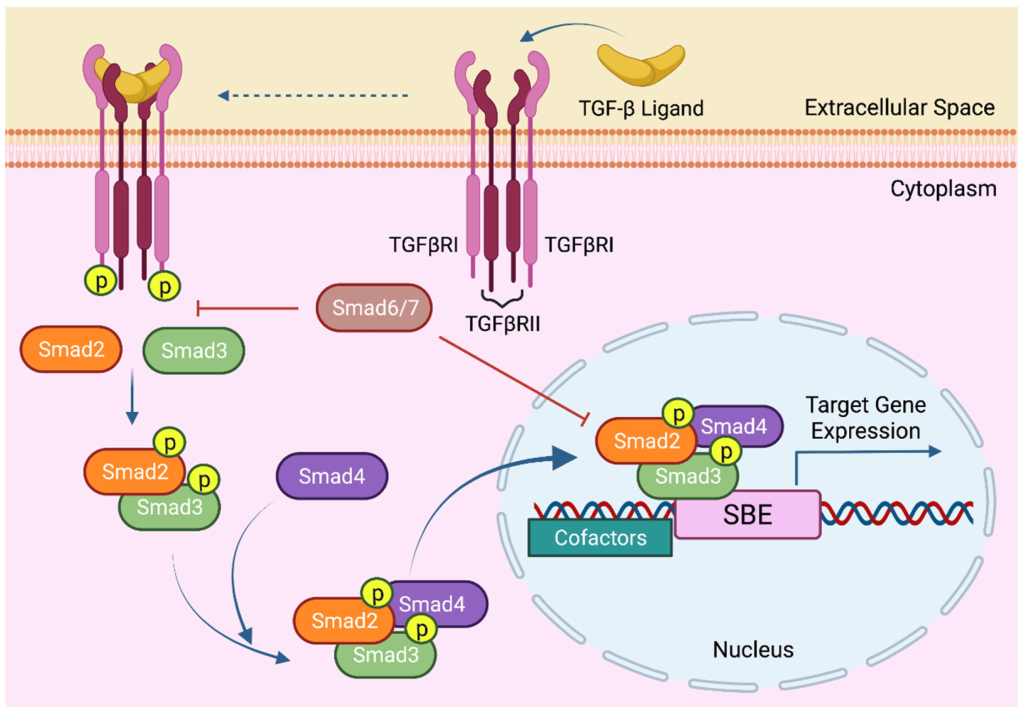
2.5.4 What are Smads?
Smads refer to a group of proteins which are transcription factors involved in regulating cell growth. Smads serve as a link between the extracellular signal (TGFβ) and a response in the nucleus. There are three members of the Smad family involved in the TGFβ pathway:
i. R-Smads (includes Smad1, 2 ,3, 5, 8/9)
ii. Co-Smads (common-mediator Smads and includes Smad4)
iii. I-Smads (inhibitory Smads which suppress the action of R-Smads in the nucleus and includes Smad6, 7)
2.5.5 Mutated Smad3 N-terminus is disrupted such that the nucleus localization signal (NLS) is no longer functional. Predict the cellular location of Smad3?
a. In the presence of the ligand TGFβ, Smad3 is transported to the nucleus.
b. In the presence of the ligand TGFβ, Smad3 remains in the cytoplasm.
The N-terminal portion of R-Smad contains domains including a “Mad-homology 1” (MH1) domain. Part of the MH1 domain includes a NLS (Hill, 2009; Xiao et al., 2000). The nuclear-localization signal (NLS) is exposed upon phosphorylation. Proteins targeted to the nucleus possess a NLS at the N-terminus, and a mutant version of Smad3 that possesses a nonfunctional NLS will remain in the cytoplasm.
2.6 Overview of Histidine-kinase-associated receptors
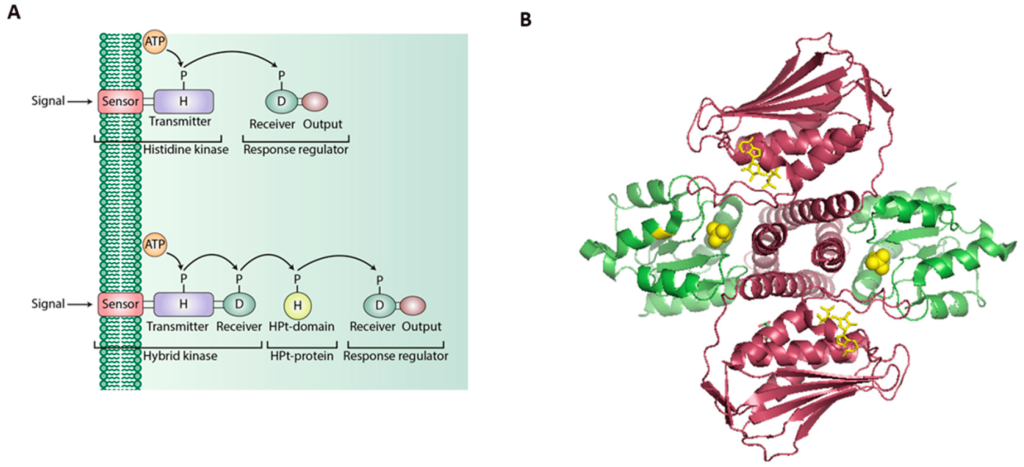
2.6.1 Describe the structure of histidine-kinase-associated receptors.
Histidine kinases transmembrane proteins dimerize upon activation. The extracellular portion contains ligand binding domains while the cytoplasmic region contains a DHp domain (Dimerization and histidine-phosphotransfer domain) along with the catalytic domain (Bhate et al., 2015; Buschiazzo & Trajtenberg, 2019). The DHp domains harbour an H box region containing the conserved His which is phosphorylated at the imidazole group (Figure 2.13). This phosphate group on His is transferred to an aspartate (Asp) on another protein, activating the protein response regulator.
Histidine kinase associated receptors are part of a ‘two-component’ cell signalling system.
2.6.2 Summarize the two-component signalling system into two discrete elements.
The two-component system is made up of two protein elements:
I. Sensor (containing an input domain [for ligand binding] and transmitter domain [containing the conserved His and ATP-binding/kinase domain]
II. Response regulator (containing a receiver domain with the conserved Asp and the effector or output domain)
This system is particularly important in bacteria for responding to extracellular events and mediates chemotaxis.
2.6.3 What is the underlying mechanism for how two-component systems operate?
This system is referred to as a phosphotransfer pathway. The histidine kinase is the sensor, while the effector protein as a regulator (Figure 2.14). Upon ligand binding, the receptor (i.e. the sensor) is then activated, followed by trans-phosphorylation of a conserved His residue. The response regulator will subsequently catalyze the transfer of the phosphate from the sensor onto a specific Asp (in the receiver domain of the response regulator) (Papon & Stock, 2019). Asp phosphorylation activates the effector protein, which leads to localization to the DNA and upregulation of transcription. In more
2.7 Overview of Receptor Guanylyl Cyclases
2.7.1 What are receptor guanylyl cyclases?
Consistent with other receptors such as RTKs, receptor guanylyl cyclases possess both extracellular and intracellular regions flanking a transmembrane domain. The transmembrane domain consists of an alpha helical segment, while the N-terminal extracellular region contains a ligand binding domain, and the C-terminal region contains a catalytic guanylyl cyclase domain. Guanylyl cyclase enzymatic activity converts GTP into a cyclized version – guanosine 3’,5’-cyclic monophosphate (cGMP) (Figure 2.15).
2.7.2 Predict the impact of incubation with phosphodiesterase on the levels of cellular cGMP.
a. Production of cGMP would decrease
b. Production of cGMP would increase
c. No effect on cGMP production
The enzyme phosphodiesterase catalyzes conversion of cGMP to 5’-GMP. The nucleotide cGMP is an example of a secondary messenger (Figure 2.15). The levels of cGMP decrease in the presence of phosphodiesterase.
Note, cGMP forms a complex with cGMP-dependent protein kinase G (PKG). PKG is activated and proceeds to phosphorylate target proteins potentially on Ser/Thr residues.
2.8 Receptor-like Tyrosine phosphatase
2.8.1 Describe the structure of receptor-like protein tyrosine phosphatases.
Similar to their counterparts (RTKs), the receptor-like protein tyrosine phosphatases are embedded within the plasma membrane with additional domains in the extracellular environment and in the cytosol. The extracellular region is variable, containing sequences similar to immunoglobulin-like motifs, carbonic anhydrase domains, or fibronectin type III-like domains (Xu & Fisher, 2012). The protein tyrosine phosphatase (PTP) domain is found in the C-terminal region and situated in the cytosol. There may be one or two copies of the PTP domain, which include a key Cys residue, essential for dephosphorylation, and the PTP domain proximal to the membrane is catalytically active, with the second domain demonstrating regulatory roles.
2.8.2 How can receptor-like tyrosine phosphatases play a role in regulating signalling pathways?
Broadly, receptor-like tyrosine phosphatases carry out dephosphorylation of tyrosine residues on target proteins (e.g. receptors with phosphotyrosine sites) which opposes the effects of kinases. For example, the MAPK pathway demonstrates the balancing act between kinases and phosphatases. Protein tyrosine phosphatase receptor type R (PTPRR) binds Erk (a MAPK). Erk is phosphorylated on both Tyr and Thr, resulting in activation of the kinase. PTPRR can counteract the kinase activity of Erk, by removing the phosphate groups (Xu & Fisher, 2012) which modulates Erk kinase activity.
2.8.3 A receptor-like tyrosine phosphatase was incubated under oxidizing conditions. Predict the downstream outcome:
a. Receptor-like tyrosine phosphatase activity would be inhibited.
b. Receptor-like tyrosine phosphatase activity would be activated.
Under oxidizing conditions, if the active site Cys is converted to sulphenic acid it will no longer be able to act as a strong nucleophile and remove the phosphate group (Xu & Fisher, 2012). It is predicted that the activity of Receptor-like tyrosine phosphatases would be inhibited.
References
Alberts, Bruce. (2002). Molecular biology of the cell. In Molecular biology of the cell (4th ed.). Garland Science. https://www.ncbi.nlm.nih.gov/books/NBK26822/
Bajusz, D., Pándy-Szekeres, G., Takács, Á., de Araujo, E. D., Erdogan, F., Neubauer, H. A., Meneksedag-Erol,raujo, E. D., & Keserű, G. M. (2023). SH2db, an information system for the SH2 domain. Nucleic Acids Research, 51(W1), W542–W552. https://doi.org/10.1093/nar/gkad420
Bhate, M. P., Molnar, K. S., Goulian, M., & DeGrado, W. F. (2015). Signal Transduction in Histidine Kinases: Insights from New Structures. Structure, 23(6), 981–994. https://doi.org/10.1016/j.str.2015.04.002
Bhagirath, A. Y., Li, Y., Patidar, R., Yerex, K., Ma, X., Kumar, A., & Duan, K. (2019). Two Component Regulatory Systems and Antibiotic Resistance in Gram-Negative Pathogens. International Journal of Molecular Sciences, 20(7). https://doi.org/10.3390/ijms20071781
Buday, L., Egan, S. E., Rodriguez Viciana, P., Cantrell, D. A., & Downward, J. (1994). A complex of Grb2 adaptor protein, Sos exchange factor, and a 36-kDa membrane-bound tyrosine phosphoprotein is implicated in ras activation in T cells. Journal of Biological Chemistry, 269(12), 9019–9023. https://doi.org/10.1016/S0021-9258(17)37070-9
Bulavin, D. V, Saito, S., Hollander, M. C., Sakaguchi, K., Anderson, C. W., Appella, E., & Fornace, A. J. (1999). Phosphorylation of human p53 by p38 kinase coordinates N‐terminal phosphorylation and apoptosis in response to UV radiation. The EMBO Journal, 18(23), 6845–6854. https://doi.org/10.1093/emboj/18.23.6845
Buschiazzo, A., & Trajtenberg, F. (2019). Two-Component Sensing and Regulation: How Do Histidine Kinases Talk with Response Regulators at the Molecular Level? Annual Review of Microbiology, 73(1), 507–528. https://doi.org/10.1146/annurev-micro-091018-054627
de Araujo, E. D., Erdogan, F., Neubauer, H. A., Meneksedag-Erol, D., Manaswiyoungkul, P., Eram, M. S., Seo, H. S., Qadree, A. K., Israelian, J., Orlova, A., Suske, T., Pham, H. T. T., Boersma, A., Tangermann, S., Kenner, L., Rülicke, T., Dong, A., Ravichandran, M., Brown, P. J., … Gunning, P. T. (2019). Structural and functional consequences of the STAT5BN642H driver mutation. Nature Communications, 10(1). https://doi.org/10.1038/s41467-019-10422-7
de Araujo, E. D., Keserű, G. M., Gunning, P. T., & Moriggl, R. (2020). Targeting STAT3 and STAT5 in Cancer. Cancers, 12(8). https://doi.org/10.3390/cancers12082002
de Araujo, E. D., Orlova, A., Neubauer, H. A., Bajusz, D., Seo, H.-S., Dhe-Paganon, S., Keserű, G. M., Moriggl, R., & Gunning, P. T. (2019). Structural Implications of STAT3 and STAT5 SH2 Domain Mutations. Cancers, 11(11). https://doi.org/10.3390/cancers11111757
Erdogan, F., Radu, T. B., Orlova, A., Qadree, A. K., de Araujo, E. D., Israelian, J., Valent, P., Mustjoki, S. M., Herling, M., Moriggl, R., & Gunning, P. T. (2022). JAK-STAT core cancer pathway: An integrative cancer interactome analysis. Journal of Cellular and Molecular Medicine, 26(7), 2049–2062. https://doi.org/10.1111/jcmm.17228
Gomperts, B. D., Kramer, I. M., & Tatham, P. E. R. (2009). Signal transduction . In Signal transduction (2nd ed.). Elsevier/Academic Press
Gungor, M. Z., Uysal, M., & Senturk, S. (2022). The Bright and the Dark Side of TGF-β Signaling in Hepatocellular Carcinoma: Mechanisms, Dysregulation, and Therapeutic Implications. Cancers, 14(4). https://doi.org/10.3390/cancers14040940
Hill, C. S. (2009). Nucleocytoplasmic shuttling of Smad proteins. Cell Research, 19(1), 36–46. https://doi.org/10.1038/cr.2008.325
Ichijo, H., Nishida, E., Irie, K., ten Dijke, P., Saitoh, M., Moriguchi, T., Takagi, M., Matsumoto, K., Miyazono, K., & Gotoh, Y. (1997). Induction of Apoptosis by ASK1, a Mammalian MAPKKK That Activates SAPK/JNK and p38 Signaling Pathways. Science, 275(5296), 90–94. https://doi.org/10.1126/science.275.5296.90
Lemmon, M. A., & Schlessinger, J. (2010). Cell Signaling by Receptor Tyrosine Kinases. Cell, 141(7), 1117–1134. https://doi.org/10.1016/j.cell.2010.06.011
Meister, M., Tomasovic, A., Banning, A., & Tikkanen, R. (2013). Mitogen-Activated Protein (MAP) Kinase Scaffolding Proteins: A Recount. International Journal of Molecular Sciences, 14(3), 4854–4884. https://doi.org/10.3390/ijms14034854
Moon, S. Y., Kim, K. D., Yoo, J., Lee, J.-H., & Hwangbo, C. (2021). Phytochemicals Targeting JAK–STAT Pathways in Inflammatory Bowel Disease: Insights from Animal Models. Molecules, 26(9). https://doi.org/10.3390/molecules26092824
Morrison, D. K. (2001). KSR: a MAPK scaffold of the Ras pathway? Journal of Cell Science, 114(9), 1609–1612. https://doi.org/10.1242/jcs.114.9.1609
Morrison, D. K. (2012). MAP kinase pathways. Cold Spring Harbor Perspectives in Biology, 4(11), a011254–a011254. https://doi.org/10.1101%2Fcshperspect.a011254.
Osaki, L. H., & Gama, P. (2013). MAPKs and Signal Transduction in the Control of Gastrointestinal Epithelial Cell Proliferation and Differentiation. International Journal of Molecular Sciences, 14(5), 10143–10161. https://doi.org/10.3390/ijms140510143
Papon, N., & Stock, A. M. (2019). Two-component systems. Current Biology, 29(15), R724–R725. https://doi.org/10.1016/j.cub.2019.06.010
Pike, L. J., Eakes, A. T., & Krebs, E. G. (1986). Characterization of affinity-purified insulin receptor/kinase. Effects of dithiothreitol on receptor/kinase function. Journal of Biological Chemistry, 261(8), 3782–3789. https://doi.org/10.1016/S0021-9258(17)35716-2
Reich, N. C. (2013). STATs get their move on. JAK-STAT, 2(4), e27080. https://doi.org/10.4161/jkst.27080
Robinson, D. R., Wu, Y.-M., & Lin, S.-F. (2000). The protein tyrosine kinase family of the human genome: Tyrosine Kinases. Oncogene, 19(49), 5548–5557. https://www.nature.com/articles/1203957
S. Corbalan-Garcia S.-S. Yang, K. R. D., & Bar-Sagi, D. (1996). Identification of the Mitogen-Activated Protein Kinase Phosphorylation Sites on Human Sosl That Regulate Interaction with Grb2. Molecular and Cellular Biology, 16(10), 5674–5682. https://doi.org/10.1128/MCB.16.10.5674
Sayem, A. S. M., Arya, A., Karimian, H., Krishnasamy, N., Ashok Hasamnis, A., & Hossain, C. F. (2018). Action of Phytochemicals on Insulin Signaling Pathways Accelerating Glucose Transporter (GLUT4) Protein Translocation. Molecules, 23(2). https://doi.org/10.3390/molecules23020258
Ségaliny, A. I., Tellez-Gabriel, M., Heymann, M.-F., & Heymann, D. (2015). Receptor tyrosine kinases: Characterisation, mechanism of action and therapeutic interests for bone cancers. Journal of Bone Oncology, 4(1), 1–12. https://doi.org/10.1016/j.jbo.2015.01.001
Tato, I., Bartrons, R., Ventura, F., & Rosa, J. L. (2011). Amino Acids Activate Mammalian Target of Rapamycin Complex 2 (mTORC2) via PI3K/Akt Signaling. The Journal of Biological Chemistry, 286(8), 6128–6142. https://doi.org/10.1074/jbc.m110.166991
Waksman, G., Shoelson, S. E., Pant, N., Cowburn, D., & Kuriyan, J. (1993). Binding of a high affinity phosphotyrosyl peptide to the Src SH2 domain: Crystal structures of the complexed and peptide-free forms. Cell, 72(5), 779–790. https://doi.org/10.1016/0092-8674(93)90405-F
Xiao, Z., Liu, X., Henis, Y. I., & Lodish, H. F. (2000). A Distinct Nuclear Localization Signal in the N Terminus of Smad 3 Determines Its Ligand-Induced Nuclear Translocation. Proceedings of the National Academy of Sciences – PNAS, 97(14), 7853–7858. https://doi.org/10.1073/pnas.97.14.7853
Xu, Y., & Fisher, G. J. (2012). Receptor type protein tyrosine phosphatases (RPTPs) – roles in signal transduction and human disease. Journal of Cell Communication and Signaling, 6(3), 125–138. https://doi.org/10.1007/s12079-012-0171-5
Yoon, M.-S. (2017). The Role of Mammalian Target of Rapamycin (mTOR) in Insulin Signaling. Nutrients, 9(11), 1176-. https://doi.org/10.3390/nu9111176
Zheng, C. F., & Guan, K. L. (1993). Properties of MEKs, the kinases that phosphorylate and activate the extracellular signal-regulated kinases. The Journal of Biological Chemistry, 268(32), 23933–23939. https://www.jbc.org/article/S0021-9258(20)80474-8/pdf
Zhou, Y., Prakash, P., Gorfe, A. A., & Hancock, J. F. (2018). Ras and the Plasma Membrane: A Complicated Relationship. Cold Spring Harbor Perspectives in Medicine, 8(10). https://doi.org/10.1101/cshperspect.a031831

