1.1 Claytonia Virginica
Amy Turnbull
Claytonia Virginica
Author: Amy Turnbull
1. Plant Description
Claytonia virginica is also known as Virginia Spring Beauty or Fairy Spuds due to the tiny edible corm.
This plant is a:
- Perennial: the plant re-grows each year from the same root
- Eudicot: the plant has two seed leaves, although in this genus, only one is dominant in the seed
- Angiosperm: this plant flowers and produces seed
- Herbaceous: no woody tissues
- Determinate growth: The stems are terminated by a bud. In the case of floral stems, floral buds form. Leaves emerge directly form the root.
- Spring ephemeral: A loose collection of plants with similar life cycles. Its life cycle occurs prior to deciduous trees leafing out when the forest floor receives sunlight. In Carolinian Canada, this is typically in April-early May (Muma, n.d.).
2. Identification
Location: This plant is found in moist woods and semi-shade meadows (Newcomb, 1989).
Flowering: Flowering occurs in April in SW Ontario. The early flowering and distinctive pink stripes on the petals of most flowers are unique. Flowers are regular symmetry with two bracts, five petaloids and five pink stamens (Figure 1.1). Petaloids look like petals but are derived from different tissue (Milby, 1980). Flowers contain both male and female organs. The style has three parts connected to a six-part ovary. Petals are white with fine pink stripes, ranging from pale to bright pink. Flowers close in darkness and during heavy cloud cover. Flowers are upright when open and nod when closed (Hilty, n.d.).
Leaves: Basal leaves arise singly from the base of the corm and taper to a petiole. Two leaves are opposite each other and about midway up the inflorescence stem and are sessile (no petiole). Distinctively, leaves are narrow, about eight times longer than wide, and are grass-like in appearance (Newcomb, 1989).
In the winter, there are no above-ground parts of the plant. It overwinters as a corm, a fleshy underground root. Leaves and flowers emerge in April. It is one of the first wildflowers to bloom in Ontario. It grows in thick stands, and its bloom can turn the forest floor shades of white and pink.

1.3 Cultivation
Seed germination: Plant in late spring outdoors in a pot. Place in a shady spot and keep moist. The plant will emerge in the following spring. It can be sealed in a bag with a moist medium at 25 C for 60 days, followed by 4 C for 60-90 d, then kept cold until planted (North Carolina State University Extension).
Location: Plant in woodlands, but it will also grow in lawns. Prefers well-drained, moist soil.
Sunlight: Does best in partial to full shade. It will grow in full sun if the soil is consistently moist.
4. Cultural History
All members of the Claytonia genus are edible. The leaves can be eaten raw and the fleshy roots can be prepared like potatoes (University of Texas, n.d.). Haudenosaunee used to consume the roots and leaves cooked. The plant grows in dense stands and would have been an important source of energy. Raw roots were consumed as a contraceptive. The plant is called meeautikwaeaugpineeg in Anishinaabemowin language (Wahid, 2014). In the woodland, it is consumed by small rodents, including chipmunks and mice.
5. Life Cycle
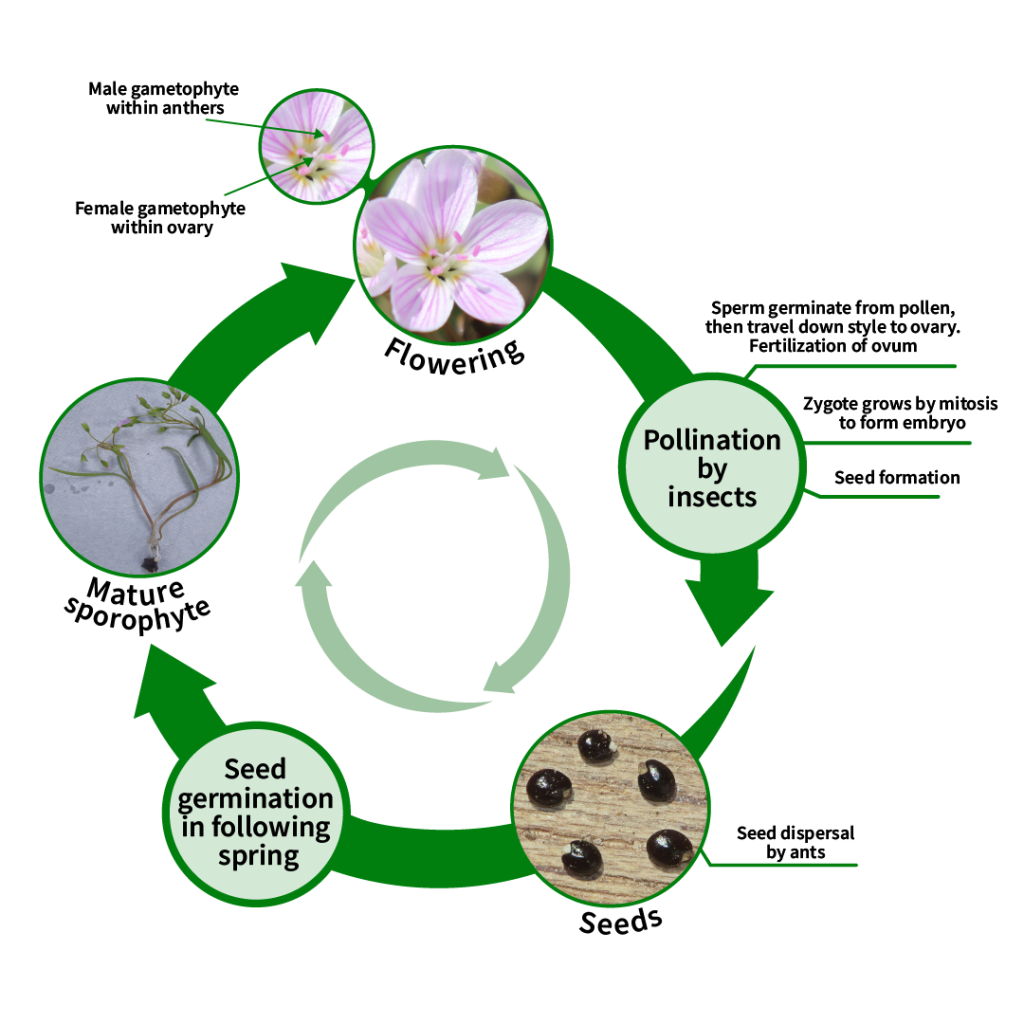
Flowering
Each flower is open for three days.
- Each stamen is only active for one day.
- The stigma is receptive to pollen for 1-2 days, depending on pollination. Unpollinated flowers can remain receptive for a third day (Milby, 1980).
Meiosis
C. virginica has a complicated chromosomal diversity. It is believed that the original population had a chromosome number of n=6, from which populations of n = 7 and 8 arose, giving rise to n = 12, 14 and 16 plants through the failure of chromosome separation in meiosis. Through the loss of redundant chromosomes, significant populations of n = 11 and 15 developed. The most common population surrounding Lake Erie is n=8, with tetraploid plants south of the region. These plants have broader leaves than other, more southerly populations (Lewis et al., 1967).
Pollination
Flowers are only pollinated by insects (Motten et al., 1981). The seed set can be affected if the weather is rainy during flowering or if flowering occurs very early before pollinators have emerged. Late in the season, the plants increasingly abort ovules due to decreased light on the forest floor as trees leaf out.
Seeds
Seeds are produced within the capsule fruit after flowering. The seeds have a fleshy structure that attracts ants to increase dispersal (Handel, 1978). The plant that arises from the seed is genetically different than the mother plant on which the flower was borne.
Evolution
In angiosperms, flowers typically are formed by two whorls: calyx (sepals) and corolla (petals). Claytonia is thought to have evolved from a wind-pollinated ancestor that lost the corolla (petal whorl). On Claytonia, the non-reproductive parts of the flower are formed by a single whorl. The bracts are anatomically similar to leaves and are derived from leaf tissue (Milby, 1980).
6. Anatomy and Physiology
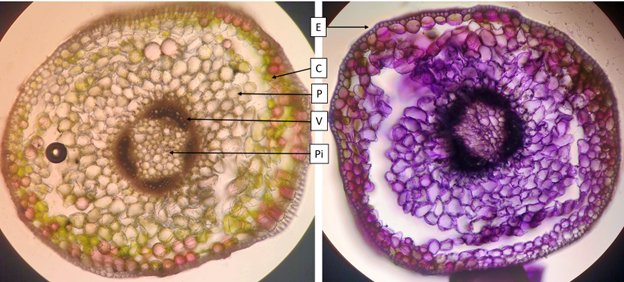
Spring ephemeral plants such as C. virginica face several challenges in their life cycle. These plants must grow rapidly and accumulate carbohydrates at an accelerated rate in order to produce mature seeds before deciduous tree leaves shade the plant (Lapointe, 2001). The plants accomplish this during the cool spring weather, when enzyme rates are low and the soil is cool (see the photosynthetic stem, Figure 1.3). Accumulated carbohydrates from photosynthesis are stored in the corm, and this allows next year’s buds to form (Figure 1.4). Once sufficient carbohydrates are accumulated, leaves senesce then roots. The main signals causing leaf senescence are increasing temperature and increasing shade from canopy closure. The plant enters dormancy until fall. Cool fall weather allows the plant to break dormancy so it can grow roots and differentiate buds for next season at a slow rate.

Nutrients are primarily absorbed during spring growth and allocated to the leaves and corm. Roots grow slowly during fall and winter and do not absorb minerals as nutrient needs are low during this time. Root growth relies on stored carbohydrates from the previous season (Anderson and Eickmeier, 2000).

As these plants must grow rapidly, they have a high photosynthetic rate (see the leaf dissection in Figure 1.5). Photosynthetic enzymes are made early in leaf development so the leaves are at full photosynthetic capacity once the leaves expand. Since photosynthesis uses water, these plants have an increased water demand in the leaves compared to similarly sized plants. The high photosynthetic rate also means increased gas exchange through the stomata, resulting in increased loss of water through transpiration. This would result in water stress for most plants, however, spring ephemerals grow when the soil is moist from snowmelt. C. virginica grows long root hairs to compensate (Lapointe, 2001).

Once the flower senesces, the petals fall, but the bracts persist (Figure 1.6). The capsule fruit matures within the bracts. As the fruit matures in 10 days, it dehisces to disperse the small, black seeds. Seeds can be dispersed many centimetres from the parent plant. Ants are attracted to the seed’s elaiosome and have been shown to disperse the seeds further for C. virginica (Handel, 1978). To maintain viability, the seeds cannot dry out. This reflects the moist woodland habitat of the species. In contrast, seeds from western Claytonia species from areas that experience regular drought can dry out. Seeds are doubly dormant and require a warm, moist period followed by a cold, moist period. Seed that is produced on a plant this year will germinate next spring.

Flower colour varies in C. virginica from pale white to mauve (Figure 1.7). The colour intensity and thickness of pink striping on the white background change the overall colour of the petaloid. Floral pigmentation is due to cyanidin, a crimson pigment molecule, and two flavenols, quercetin and kaempferol. As the concentration of flavenols increases, the flower becomes whiter; as their concentration decreases, the flower becomes redder.
Increased flower redness resulted in increased pollination by insects and increased seed production. White flowers are rare and have the lowest seed production due to pollinators. The effect of pollinators is amplified when C. virginica is surrounded by other species of white-flowering plants. When a plant has a distinct ‘floral signal’ (colour in this case), pollinators are less likely to move between species and are more likely to stay within the species with the strong signal to increase their reward.
Herbivore damage and fungal rust infection are related to flower colour, affecting reproductive fitness. Plants that have leaves removed by herbivores are more likely to die, and those that survive will produce smaller leaves the following year. As concentrations of the two flavanols increase, flowers become whiter, and herbivore damage decreases (Frey, 2004). Leaves of red-flowered plants were more likely to be eaten by herbivores (Frey, 2004). This was speculated to increase general defence compounds, which decreased fungal lesions. Thus, red plants were less likely to have fungal lesions as they were expressing antifungal defence compounds resulting from herbivore attacks.
C. virginica has two important pollinators.
- The specialist Andrena erigeniae (Spring Beauty Mining Bee) visits the flowers for pollen. The bees are 8 mm long, the same size as the flower’s diameter.
- The generalist Bombylis major (greater bee fly). The bee fly visits many species of spring flowers for nectar and also transfers pollen in the process. Bee flies are 12-18 mm large, about twice the size of the flowers. Bee flies make less contact with flowers than the Spring Beauty Miner bees do, owing to their long mouth part that sucks nectar with minimal flower contact. Both pollinators occur in Ontario.
7. Summary
This small plant has a fascinating history with its variable petal colouration and ant-attracting seeds. It is easily enjoyed in nature and the garden through its early flowering.
References
Anderson, W. B., & Eickmeier, W. G. (2000). Nutrient resorption in Claytonia virginica L.: Implications for deciduous forest nutrient cycling. Canadian Journal of Botany, 78(6), 832–839. https://doi.org/10.1139/b00-056
Frey, F. M. (2004). Opposing natural selection from herbivores and pathogens may maintain floral-color variation in Claytonia virginica (Portulacaceae). Evolution, 58(11), 2426. https://doi.org/10.1554/03-477
Handel, S. N. (1978). New ant-dispersed species in the genera Carex, Luzula, and Claytonia. Canadian Journal of Botany, 56(22), 2925–2927. https://doi.org/10.1139/b78-351
Hilty, J. (n.d.). Spring beauty (Claytonia virginica). Illinois Wildflowers. https://www.illinoiswildflowers.info/woodland/plants/spring_beauty.htm
Lapointe, L. (2001). How phenology influences physiology in deciduous forest spring ephemerals. Physiologia Plantarum, 113(2), 151–157. https://doi.org/10.1034/j.1399-3054.2001.1130201.x
Lewis, W. H., Oliver, R. L., & Suda, Y. (1967). Cytogeography of Claytonia virginica and its allies. Annals of the Missouri Botanical Garden, 54(2), 153. https://doi.org/10.2307/2395001
Milby, T. H. (1980). Studies in the floral anatomy of Claytonia (Portulacaceae). American Journal of Botany, 67(7), 1046–1050. https://doi.org/10.1002/j.1537-2197.1980.tb07736.x
Motten, A. F., Campbell, D. R., Alexander, D. E., & Miller, H. L. (1981). Pollination effectiveness of specialist and generalist visitors to a North Carolina population of Claytonia virginica. Ecology, 62(5), 1278–1287. https://doi.org/10.2307/1937292
Muma, W. (n.d.). Narrow-leaved spring beauty Claytonia virginica. Ontario Wildflowers. http://ontariowildflowers.com/main/species.php?id=178
Newcomb, L. (1989). Wildflowers with opposite leaves. In Newcomb’s wildflower guide (p. 270). Little, Brown.
North Carolina State University Extension. (n.d.). Claytonia virginica. Claytonia virginica (Common Spring-beauty, Fairy Spud, Spring Beauty, Spring-beauty) | North Carolina Extension Gardener Plant Toolbox. https://plants.ces.ncsu.edu/plants/claytonia-virginica/
University of Texas at Austin. (n.d.). Spring beauty. Texas Beyond History. https://www.texasbeyondhistory.net/coast/nature/images/spring-beauty.html
Wahid, Z. (2014, June 10). Claytonia virginica spring beauty. [PDF]. University of Michigan. https://deepblue.lib.umich.edu/bitstream/handle/2027.42/109732/Wahid_Zachariah_2014.pdf?sequence=1


