Experiment 4: pH Adjustment
Purpose
- Determine the operating conditions which provide good control in the neutralization of a waste stream.
- Examine the effects of control bandwidth, reagent strength, and reagent pumping rate on the neutralization of a waste stream.
Introduction
The effluent from an industrial process will often contain a diverse array of contaminants and may exhibit a complex set of transient characteristics. For example, the wastewater might be contaminated by heavy metals, oils & greases or solvents, the pH might range anywhere from 0-14, the solution might be a strong oxidant or a reductant. As a result, depending on the specific characteristics of the waste, the effluent might require several different types of treatment: metals removal, BOD control, emulsion breaking, cyanide destruction, etc. In virtually all situations, however, the treatment of industrial effluent will require pH adjustment to near neutral conditions prior to sewer discharge. This unit process is termed Neutralization.
Neutralization typically involves the addition of acid and/or base reagents to the wastewater, via chemical feed pumps under the control of a pH indicator-Controller (pH I/C). The operation is normally executed in a stirred reactor, either on a batch or continuous-flow basis. The pH I/C monitors the pH of the waste solution and, if the level is above or below pre-determined control values, activates the appropriate reagent feed pump. The reagent pump is maintained in an active condition until the target pH level is attained. As such, Neutralization would appear to be a relatively uncomplicated operation and easy to execute. In practice, however, the process is subject to a number of complications:
- Under most conditions, there will be a lag between the addition of a reagent and the response of the pH I/C to this addition. As a result, the pump will continue to operate for a period of time longer than necessary and the volume of reagent injected will exceed the quantity required. In applications where concentrated reagents are used, it is possible that this excess of acid or base will cause the pH to jump beyond the opposite control point. In response, the system will initiate the feed of the other reagent and this might, in turn, cause the pH to move back outside the initial control value. In extreme situations, the system may loop back and forth indefinitely, resulting in excessive reagent consumption and increased equipment wear and tear.
- While the situation described above would tend to argue in favour of the use of dilute reagents, this option may also yield unacceptable results. In continuous flow systems, the residence time in the reactor is the quotient of the tank volume and the solution flow rate:
[latex]\tau = \frac{V}{Q}[/latex]
where:
τ = residence time, min
V = tank volume, m3
Q = flow rate, m3/minThe pH adjustment operation must be fully completed while the slug of effluent is in the reactor and, as a result, the reagent must have sufficient strength to complete the pH change within this period of time. Therefore, the optimum reagent strength must be a compromise between these two extremes.
- In many individual applications, the pH of the incoming effluent may be highly variable. For example, the wastewater pH might be 6.4 for several minutes while a specific set of processes are active. Suddenly, a cleaning line might discharge some spent wash water, causing the pH to jump to 11.3. Shortly after this, an acid etch might discharge waste at a pH of 2.6 to the system. The pH control system must be capable of accommodating both moderate and extreme conditions.
- Most Sewer-use bylaws will allow effluent discharges within a broad range of pH values, typically 6.0 to 10.0. As a result, if the only requirement is compliance with the sewer limits, it may be sufficient to set the pH I/C control points at say 7.0 and 9.0. However, if the neutralization operation is to be followed by some pH-dependent process, such as metals precipitation, it may be necessary to exercise considerably tighter control over the pH range. For copper hydroxide precipitation, for example, the pH should be maintained between pH values of 8.5 and 9.0.
In most situations, it will be necessary to select a pump feed rate (mL/min or USGPH), a reagent concentration, and an acceptable span of control points which will provide the required quality of final discharge, economically and reliably.
Figure 1 below shows a typical setup for a pH adjustment with process control.
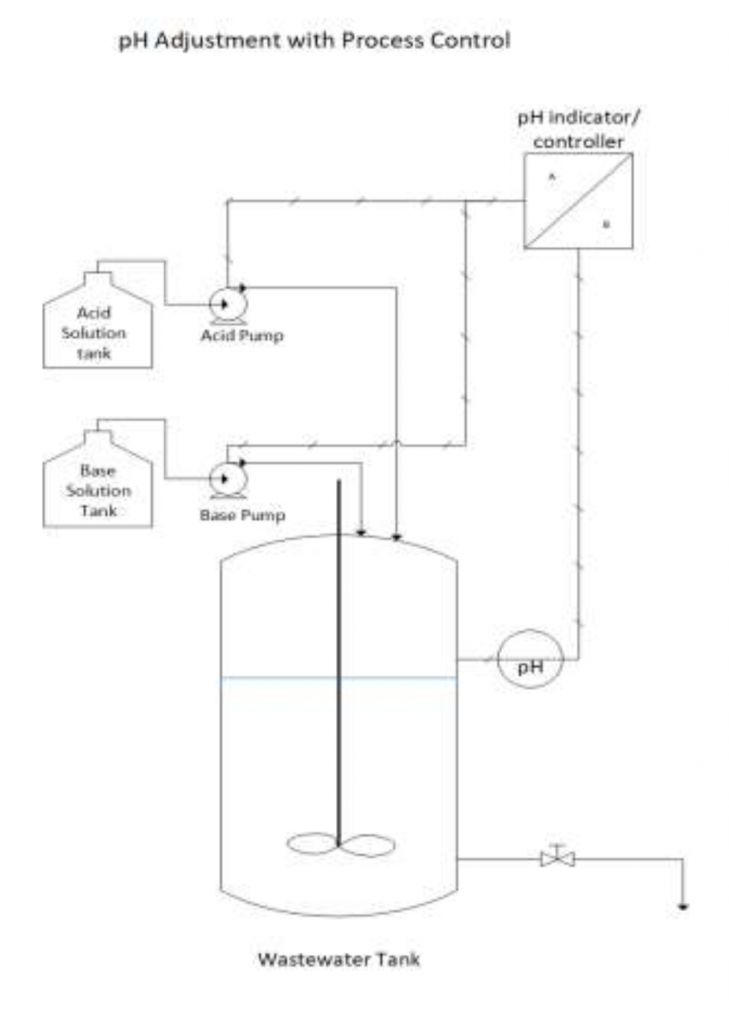
Figure 1: Typical setup of a pH adjustment with process control
Figure 2 shows the equipment used in the lab with most of the critical elements highlighted.
Additional Information:
For additional information about pH adjustment watch the video below or visit the website provided.
For more information about pH adjustment, pH controllers, and usages visit https://encyclopedia.che.engin.umich.edu/ph-measurement/.
Neutralization Video
Watch the video below for more information about the experiment and the procedures in the lab. (The link would open a new tab for the video).
Experiment Video: pH Adjustment/Neutralization
System Description
In Lab E030, pH adjustment and process control is achieved through the use of Prominent® indicators/controllers and pumps. The settings on the controller are achieved through a very user-friendly menu summarized below:
 Figure 2: DACb indicator/controller set to pH 6-8 |
 Figure 3: Pump setting for control trial |
For pH control with Prominent® DAC indicator/controller
The STOP/START on the controller can be used at any point in a run but should be in the STOP mode any time adjusting the controls.
To set a single pH set point
Menu → Control → 1 Channel parameters set 1 → Set point → Set the pH using up/down arrows → Press OK → Continue to pres ESC key to go to the main screen
To set a range of pH set points
Menu → Control → 2 Channel parameters set 1 → Lower Set point → Set the pH → Press OK → Lower Set point → Press OK → Continue to pres ESC key to go to the main screen
To set a single pH set point or a pH range of set points
Menu → Control → Type PID 1-way or PID 2-way
For pump operation with Prominent® beta/4 pump
Operation without controller
The large white dial is for stroke length, the small white dial is for frequency. Both of these dials must be set to a value on the pump face for it to operate properly.
Operation with controller
The large white dial needs to be set to the desired stroke length, and the small white dial for 'EXTERN'. In between runs, the small white dial should be in the 'stop' mode.
Check Your Understanding
You can try the following questions for practice about pH adjustment and the equipment to be used for the experiment:
Prelab: pH Adjustment
Generate the following data tables in your lab notebook:
Table 1: Pump Calibration
| Pump Settings (stroke length/frequency) |
Volume Water Pumped (mL) |
Time of Pumping (s) |
Flow Rate (mL/s or mL/min) |
| Acid | |||
| Acid | |||
| Base | |||
| Base |
Table 2: Neutralization Data
| Run 1 | Run 2 | Run 3 | Run 4 | |
| Acid Pump: Base Pump: Acid Conc: Base Conc: |
Acid Pump: Base Pump: Acid Conc: Base Conc: |
Acid Pump: Base Pump: Acid Conc: Base Conc: |
Acid Pump: Base Pump: Acid Conc: Base Conc: |
|
| Time | pH | pH | pH | pH |
Experimental Procedure
- Calibrate both the acid and base metering pumps:
- Insert the inlet line into the large bucket of water
- Insert the outlet line into the 100 mL graduated cylinder
- Set the large dial on the pump face (% stroke length) to 30%
- Set the small dial on the pump face (stroke frequency) to 100%
- Run a timer for 3 to 5 minutes and record the volume of water collected in the cylinder; convert this volume and time to a flow rate
- Repeat this procedure for a second stroke length or frequency (60% for the large dial, 100% for the small dial)
- Fill the tank with tap water (if not already full). The tank should be filled to about 40% to 50% of the full depth. Start the mixer and ensure there are relatively turbulent conditions within the reactor.
- Begin the trial with the controller in the 'STOP' mode. Set the pH control bandwidth to 6.0-8.0.
- Ensure the pH probe is inserted through the lid of the tank and adjust the initial pH to about 5 by adding acid OR a pH of 9 by adding a base.
- Insert the line to the pumps into the first set of reagents (2% acid and 2% base).
Note: The acid and base lines should be purged from the previous solution. To do that, run the pumps manually with new solutions for around 1 minute.
Start with the pump setting to 40% stroke length for each reagent and the small dial set to 'EXTERN'; this setting will cause the pump to work through the controller to add acid or base as need be to reach the desired set point (pH 6 to 8).
- To start the trial, press the 'START' button again on the controller. Record the pH reading at 10-second intervals until the tank contents have been neutralized (i.e. final pH within the desired bandwidth).
Note: The tank is neutralized when the pH stays within the desired bandwidth and the acid and base pumps have remained off for at least ONE MINUTE.
- If the pumps keep cycling, stop the experiment after 10 minutes.
- Repeat steps (6) and (7) but change one pump setting to 70% for the second run. Readjust the initial pH as needed to be outside of the desired control limits.
- Repeat steps (6) to (8) but change the solutions to 5% acid and 5% base.
Note: The acid and base lines should be purged from the previous solution. To do that, run the pumps manually with new solutions for around 1 minute.
- At the end of the experiment stop the process and clean up any spills. Do not empty the contents of the tank unless explicitly asked to do so by the instructor. Return the equipment to the original conditions and locations.
Report
- For each run, prepare a plot of pH versus time.
- All runs should be plotted on one graph using a different series/symbols for each trial.
- Identify the range (upper and lower) in pH readings.
- Identify which trials ended at a steady-state pH and which did not. How long did it take for the trials that were neutralized?
- Identify the number of times the system cycled back between the upper and lower control points.
- Based on your trials, identify the optimum conditions for good control and the approximate length of time taken to complete the neutralization process.
- Describe some complications that can arise in controlling the pH of industrial wastes. Describe two specific industries where neutralization processes would commonly be used.
- What is the effect of different reagent strengths, with references to difficulties encountered in controlling the reaction?
- Calculate the amount consumed acid and base for each run.
Table 3: Summary table of the acid and base amounts consumed for each run
| Run 1 | Run 2 | Run 3 | Run 4 | |
| Parameters (stroke length, frequency, acid and base strength) |
||||
| Amount of Acid consumed (mL) | ||||
| Amount of Base consumed (mL) |
