Experiment #3: Brownian motion
1. Introduction to Brownian motion
Lab Goal: The goal of this experiment is to reproduce the 1909 observations on the Brownian motion of latex beads made by Jean Perrin, which are considered to have definitively proven the existence of molecules, and for which he earned the Nobel Prize in Physics in 1926.
1. Introduction
1.1 Einstein’s predictions about the Brownian motion of small particles
In one of his famous 1905 papers, Albert Einstein made a number of predictions about the Brownian motion of small particles.
He started from the hypothesis that matter is made of molecules, and that Brownian motion is caused by collisions with solvent molecules. As such, he predicted that a particle submitted to Brownian motion should have a mean-squared displacement that increases linearly with time:
(1) ![]()
where ![]() is the dimensionality of the motion (
is the dimensionality of the motion (![]() for 3D diffusion, and
for 3D diffusion, and ![]() for 2D diffusion), and where
for 2D diffusion), and where ![]() is the diffusion coefficient, a constant parameter characterizing the diffusion of the particle.
is the diffusion coefficient, a constant parameter characterizing the diffusion of the particle.
He further predicted that the diffusion coefficient of a spherical particle of radius ![]() will depend on the viscosity (
will depend on the viscosity (![]() ) and absolute temperature (
) and absolute temperature (![]() , measured in Kelvin) of the solvent, according to the following equation (know as the Stokes-Einstein relationship):
, measured in Kelvin) of the solvent, according to the following equation (know as the Stokes-Einstein relationship):
(2) ![]()
In Eq. 2, ![]() is Boltzmann’s constant, which is related both to the gas constant,
is Boltzmann’s constant, which is related both to the gas constant, ![]() ,and to the number of molecules in a mole of fluid (Avogadro’s number),
,and to the number of molecules in a mole of fluid (Avogadro’s number), ![]() , where
, where ![]() . Eq. 1 can therefore be rewritten as:
. Eq. 1 can therefore be rewritten as:
(3) ![]()
In 1905, however, both the values of ![]() and
and ![]() were unknown.
were unknown.
1.2 Perrin’s experiments
Jean Perrin set out to verify Einstein’s predictions by observing latex beads of known sizes (in the range 0.1 to 5 ![]() m) diffusing in solutions of varying viscosities. Using a light microscope, he was able to:
m) diffusing in solutions of varying viscosities. Using a light microscope, he was able to:
- Determine individual bead trajectories (as shown in Fig. 1 below).
- Check that the bead’s mean-squared displacement was indeed linear in time.
- Show that the beads’ diffusion coefficient obeyed Eq. 2 (i.e. that
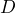 varied as expected with medium viscosity and bead size).
varied as expected with medium viscosity and bead size). - Obtain an estimate of Avogadro constant (using Eq. 3) that was remarkably close to its actual value.
This experimental confirmation that Einstein’s predictions were correct confirmed the hypothesis that matter was made of molecules.
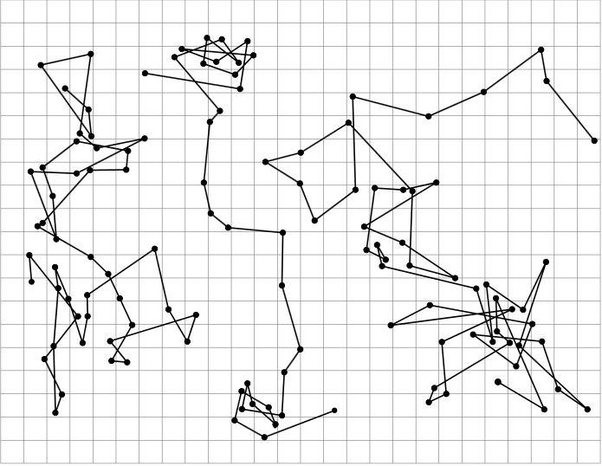 Figure 1: Three different trajectories recorded by Jean Perrin for particles of radius 0.52 μm. The dots in the trajectory show the position of the particles at 30 s intervals.The side of a square represents a 3.125 μm distance in the sample. (Reproduced from J. Perrin, Brownian Motion and Molecular Reality, Annales de Chimie et de Physique, 1909.)
Figure 1: Three different trajectories recorded by Jean Perrin for particles of radius 0.52 μm. The dots in the trajectory show the position of the particles at 30 s intervals.The side of a square represents a 3.125 μm distance in the sample. (Reproduced from J. Perrin, Brownian Motion and Molecular Reality, Annales de Chimie et de Physique, 1909.)
