10.3 Nutrient Cycles in Ecosystems
While energy flows through ecosystems in a one-way stream, matter is continuously recycled. The elements that make up living organisms, such as carbon, nitrogen, phosphorus, and water, move through the environment in complex cycles that connect the atmosphere, land, water, and living things. These are known as biogeochemical cycles, which are the natural pathways by which essential elements circulate through both the living (bio-) and non-living (geo-) parts of the Earth. Along the way, elements are stored in abiotic reservoirs (non-living parts of the environment such as the atmosphere, oceans, soil, and rocks) before being taken up again by organisms. These cycles are essential for life, as they ensure that nutrients are reused and made available across generations.
The Water Cycle
Water is essential for all living things. It plays a critical role in biological processes such as digestion, circulation, and temperature regulation. The average adult human body is about 60% water. Most land animals, including humans, require a steady supply of fresh water to survive.
Despite its importance, fresh water is surprisingly scarce. While about 2.5% of Earth’s water is freshwater, nearly all of it is locked away in glaciers, ice caps, or underground aquifers. Only about 0.5% of Earth’s total water is accessible for human use, including both surface water and groundwater that can be reached through wells. This small fraction supports most terrestrial life and ecosystems. A lack of access to this limited supply can have major effects on ecosystem dynamics and human societies. To meet growing demands, humans have developed technologies such as wells, rainwater collection, and desalination to increase water availability.
The water cycle (or hydrologic cycle) describes how water moves continuously through the environment, connecting the atmosphere, land, and bodies of water. The water cycle begins with evaporation, where heat from the sun causes water from oceans, lakes, and rivers to change into water vapour and rise into the atmosphere. Plants also contribute through transpiration, releasing water vapour from their leaves.
As water vapour rises, it cools and condenses into clouds in a process known as condensation. Eventually, the water returns to Earth as precipitation in the form of rain, snow, sleet, or hail. Some of this water flows over the land as runoff, entering rivers, lakes, and oceans. Some soaks into the ground, replenishing groundwater supplies that plants and animals depend on.
The water cycle is powered by solar energy and does not have a beginning or end. It plays a vital role in regulating climate, supporting plant growth, and shaping ecosystems. Human activities, such as deforestation, urban development, and climate change, can alter the natural flow of water and disrupt local and global ecosystems.
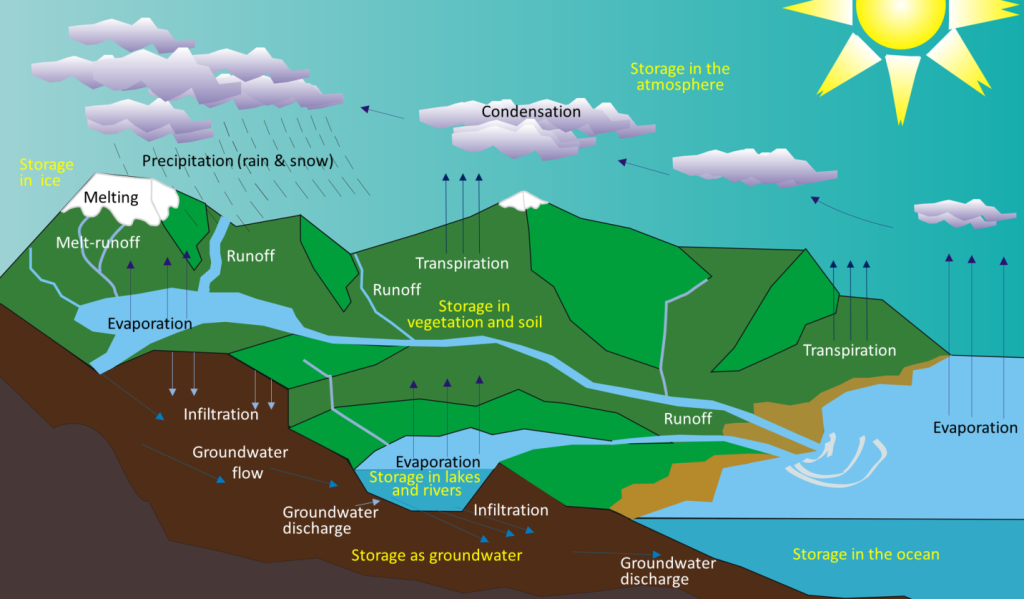
Figure 10.3.1 Image Description
The diagram illustrates the water cycle, showing how water moves through the atmosphere, land, and oceans. Starting with evaporation from oceans, lakes, and rivers, and transpiration from plants, water vapour rises into the atmosphere. It condenses into clouds (condensation) and returns to Earth as precipitation (rain or snow). In mountainous areas, snow and ice undergo melting and produce melt-runoff. Water then flows as runoff into rivers, lakes, and oceans, or infiltrates into the ground, becoming groundwater. Groundwater can move through groundwater flow and discharge back into rivers, lakes, and oceans. Water is stored in various reservoirs: ice, vegetation and soil, lakes and rivers, groundwater, oceans, and the atmosphere. The sun drives the entire process by providing energy for evaporation and transpiration.
The Carbon Cycle
Carbon is a key building block of life. It forms the backbone of important biological molecules like carbohydrates, proteins, fats, and DNA. The carbon cycle describes how carbon moves between the atmosphere, living organisms, oceans, and the Earth’s surface.
Carbon enters the ecosystem primarily through photosynthesis, when plants, algae, and some bacteria absorb carbon dioxide (CO₂) from the atmosphere and use it to build organic molecules. These molecules are then passed through the food web as organisms eat plants and each other. When organisms release energy through cellular respiration, they return CO₂ to the atmosphere. When organisms die, decomposers break down their bodies, releasing carbon back into the soil and air.
The main abiotic reservoirs for carbon are the atmosphere (as CO₂ gas), the ocean (where carbon is dissolved in water and stored in marine sediments), and fossil fuels (such as coal, oil, and natural gas, formed from ancient biological material). Carbon can remain in these reservoirs for varying lengths of time – from days to millions of years.
Human activities have significantly altered the carbon cycle. The burning of fossil fuels like coal, oil, and natural gas releases large amounts of CO₂ into the atmosphere. Deforestation also reduces the number of plants available to absorb carbon. These changes have led to rising levels of atmospheric CO₂, contributing to climate change and affecting ecosystems around the world.
The Carbon Cycle
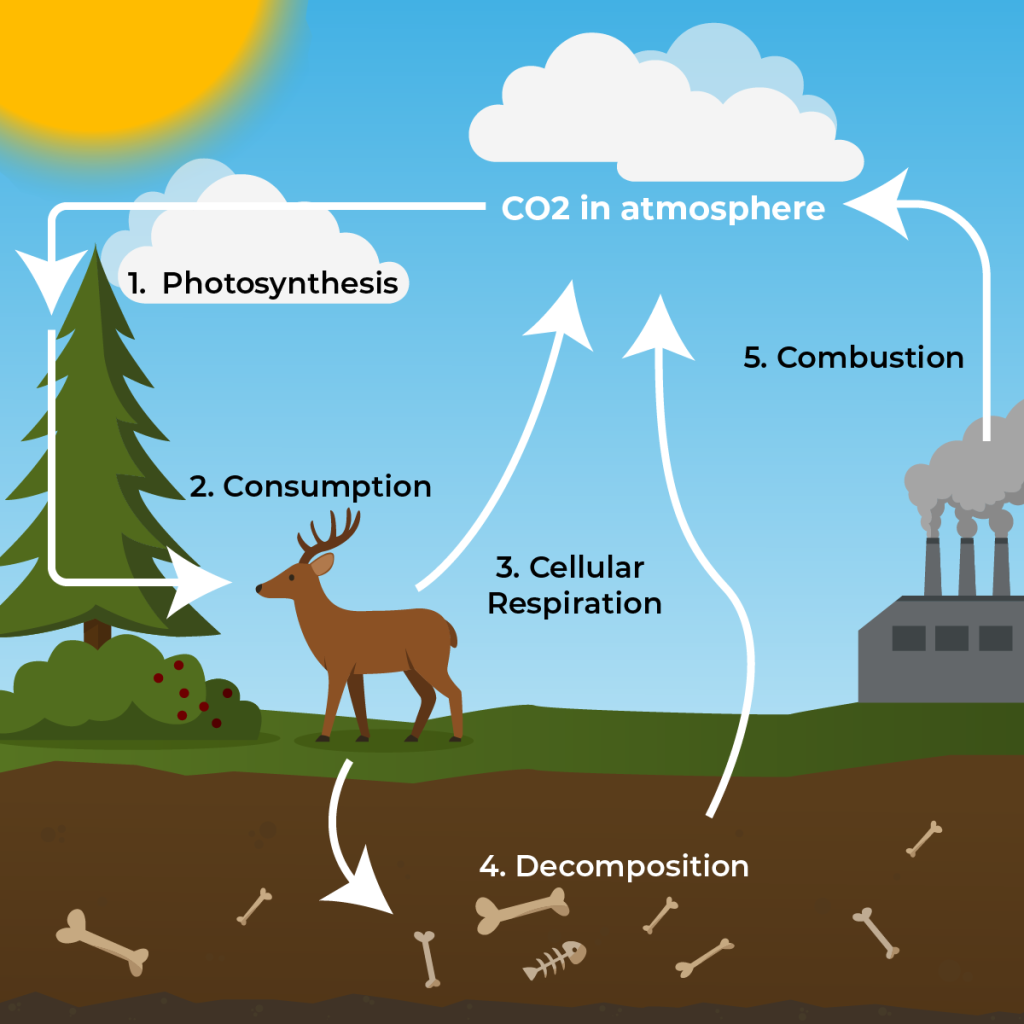
Text Description
A diagram of the carbon cycle showing how carbon moves through the environment.
- Photosynthesis: Plants, algae, and some bacteria absorb carbon dioxide (CO₂) from the atmosphere and use it to make food through photosynthesis.
- Consumption: Animals eat plants (and other animals), transferring carbon through the food web.
- Cellular Respiration: Organisms release CO₂ back into the atmosphere as a byproduct of cellular respiration.
- Decomposition: When organisms die, decomposers break down their bodies, returning carbon to the soil and air.
- Combustion: Carbon stored in fossil fuels is released into the atmosphere through human activities (like burning coal and oil).
The diagram highlights the continuous cycling of carbon between the atmosphere, plants, animals, soil, and human activities.
The Nitrogen Cycle
Nitrogen is an essential element for all living things. It is a key component of amino acids, proteins, and DNA. Although nitrogen gas (N₂) makes up about 78% of Earth’s atmosphere, most organisms cannot use it in this form. The nitrogen cycle describes how nitrogen moves between the atmosphere, soil, water, and living organisms.
The cycle begins with nitrogen fixation, a process in which certain bacteria (often found in soil or in the roots of legumes) convert atmospheric nitrogen gas into forms that plants can absorb, such as ammonium (NH₄⁺). Other bacteria in the soil carry out nitrification, converting ammonium into nitrates (NO₃⁻), which plants can use more easily.
Plants take up these nitrogen compounds from the soil and use them to build proteins and other important molecules. When animals eat plants (or other animals), nitrogen moves through the food web. When organisms excrete waste or die, decomposers break down the organic material, returning nitrogen to the soil in the form of ammonium.
Other soil bacteria perform denitrification, converting nitrates back into nitrogen gas, which is released into the atmosphere and completes the cycle.
The main abiotic reservoirs for nitrogen include the atmosphere (as N₂ gas), soil (containing ammonium and nitrate compounds), and water bodies (where nitrogen can accumulate from runoff). Human activities (such as the use of synthetic fertilizers and large-scale agriculture) have significantly altered the nitrogen cycle. Excess nitrogen from fertilizers can run off into waterways, leading to eutrophication, a process in which nutrient overload causes rapid algal growth (called algal blooms). As the algae multiply, they eventually exhaust the available nutrients and sunlight, especially in dense blooms where light can’t penetrate the water. Once the algae die, their decomposition by bacteria consumes large amounts of oxygen from the water, leading to low-oxygen conditions that can suffocate fish and other aquatic organisms.
The Nitrogen Cycle
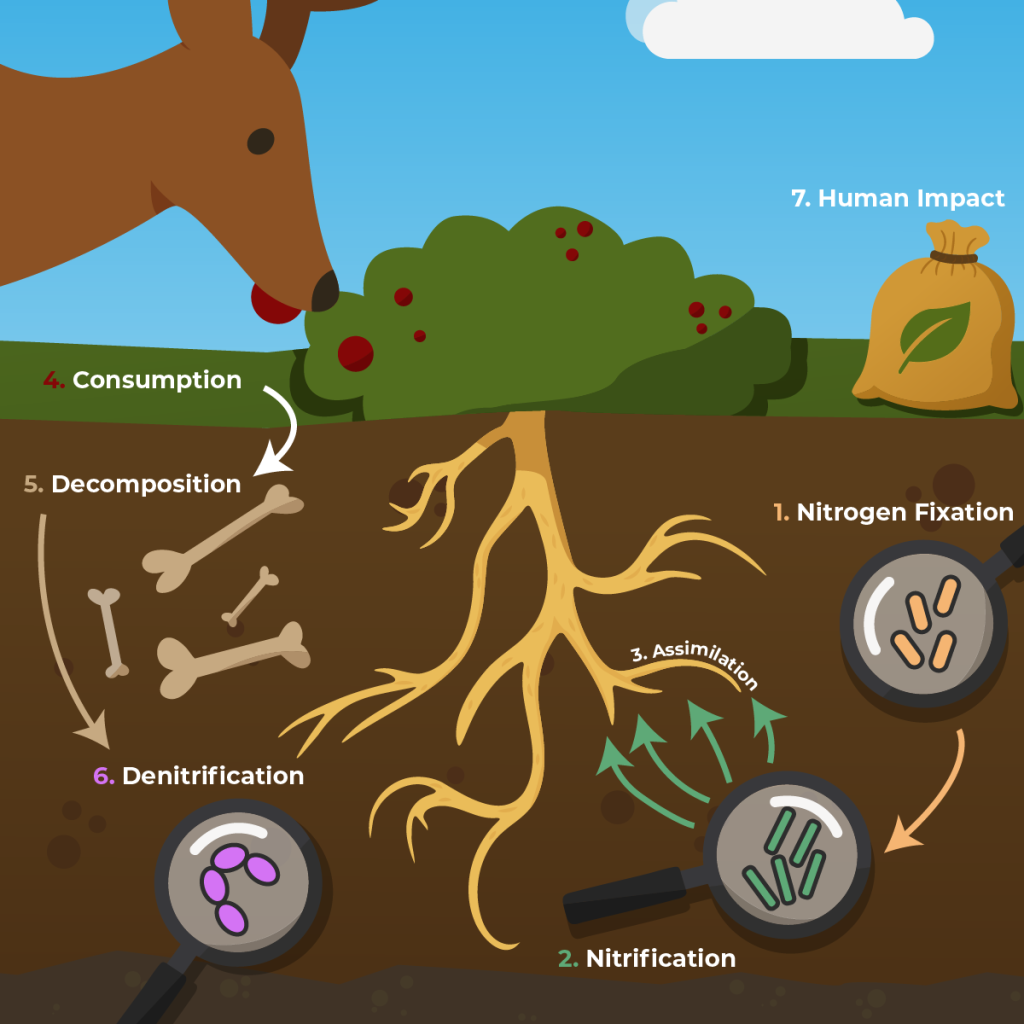
Text Description
A diagram of the nitrogen cycle showing the movement of nitrogen through the atmosphere, soil, plants, and animals. At the top, atmospheric nitrogen (N₂). The cycle contains the following steps:
- Nitrogen Fixation: Nitrogen-fixing bacteria (in soil or root nodules of legumes) convert nitrogen gas (N₂) from the atmosphere into ammonium (NH₄⁺), a form plants can use.
- Nitrification: Nitrifying bacteria convert ammonium into nitrites (NO₂⁻) and then into nitrates (NO₃⁻), which are more easily absorbed by plants.
- Assimilation: Plants absorb nitrates from the soil and use them to build proteins and other nitrogen-containing molecules.
- Animals obtain nitrogen by eating plants or other animals, moving nitrogen through the food web.
- Decomposition: Decomposers break down nitrogen-rich waste and dead organisms, returning nitrogen to the soil as ammonium
- Denitrification: Denitrifying bacteria convert nitrates in the soil back into nitrogen gas (N₂), which is released into the atmosphere.
The Phosphorus Cycle
Phosphorus is a vital nutrient for all living organisms. It is a key component of DNA, RNA, ATP, and cell membranes. Unlike carbon and nitrogen, phosphorus does not cycle through the atmosphere. Instead, it moves through the environment via rocks, soil, water, and living things.
The phosphorus cycle begins when phosphate-containing rocks are broken down by weathering. This releases phosphate ions (PO₄³⁻) into the soil and water, where they can be absorbed by plants. Plants use phosphorus to build important biological molecules, and animals obtain it by eating plants or other animals.
When organisms excrete waste or die, decomposers return phosphorus to the soil or sediments. Some of this phosphorus may be carried by runoff into rivers, lakes, and oceans, where it can settle and become part of new sedimentary rock over long periods of time.
The main abiotic reservoirs for phosphorus are rocks, soil, and sediments. Human activities, such as the use of phosphate-based fertilizers and detergents, can add excess phosphorus to waterways. Similar to the nitrogen cycle, this can lead to eutrophication and harm aquatic ecosystems.
The Phosphorus Cycle
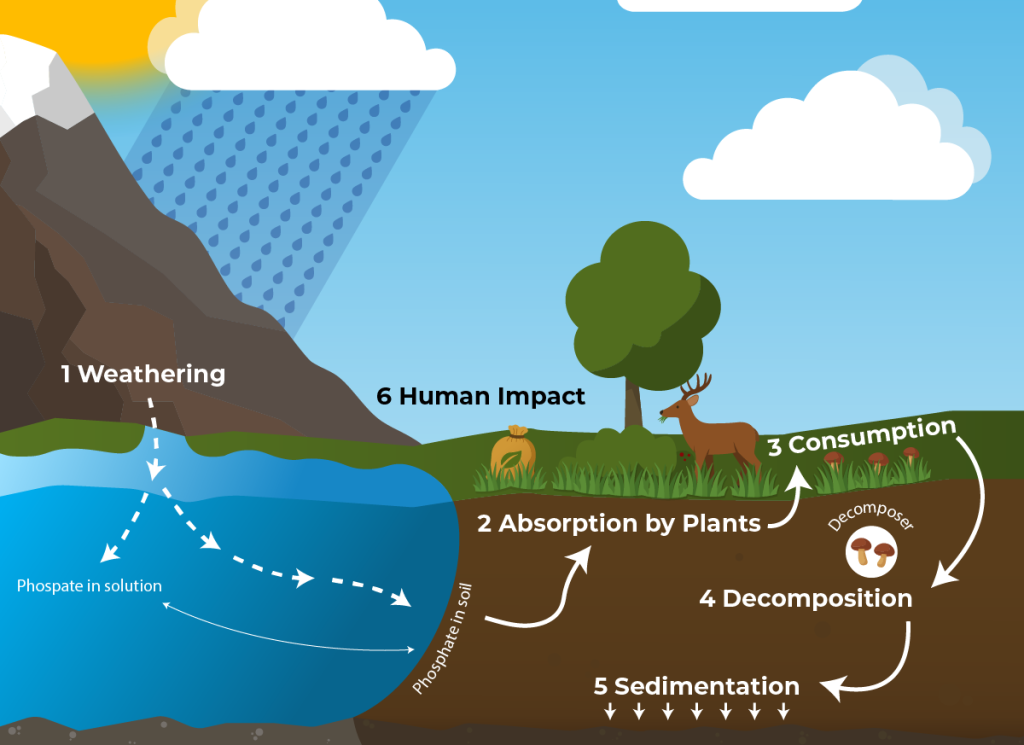
Text Description
A diagram illustrating the phosphorus cycle in an ecosystem.
- Weathering: Phosphate is released from rocks through weathering and erosion and enters the soil and water.
- Sedimentation: Phosphorus in water can settle and become part of sediments, eventually forming new rock.
- Absorption by Plants: Plants absorb phosphate from the soil and use it to build DNA, ATP, and other molecules.
- Consumption: Animals obtain phosphorus by eating plants or other animals.
- Decomposition: Decomposers break down waste and dead organisms, returning phosphorus to the soil or water.
- Human Impact: Fertilizers and detergents add phosphorus to ecosystems, often leading to water pollution and eutrophication.
The cycle shows continuous movement of phosphorus between rocks, water, soil, plants, and animals.
Knowledge Check
Text Description
- Energy is recycled while nutrients are lost
- Both energy and nutrients flow in one direction only
- Energy flows in one direction; nutrients are continuously recycled
- Nutrients flow through decomposers first; energy does not
- Cellular respiration
- Photosynthesis
- Combustion
- Decomposition
- Denitrification
- Assimilation
- Nitrogen fixation
- Nitrification
- It does not involve living organisms
- It does not cycle through the soil
- It does not cycle through the atmosphere
- It is not affected by human activities
- Acid rain
- Eutrophication
- Greenhouse effect
- Ozone depletion
Answers:
- c
- b
- c
- c
- b
OpenAI. (2025). ChatGPT. [Large language model]. https://chat.openai.com/chat
Prompt: Create 5 multiple-choice questions using the following content
“13.1 The Hydrological Cycle” from Physical Geology by Steven Earle, Earle & Steven is licensed under a Creative Commons Attribution 4.0 International License, except where otherwise noted.

