5.3 Light-Dependent Reactions
How can light be used to make food? It is easy to think of light as something that exists and allows living organisms, such as humans, to see, but light is a form of energy. Like all energy, light can travel, change form, and be harnessed to do work. In the case of photosynthesis, light energy is transformed into chemical energy, which autotrophs use to build carbohydrate molecules.

What Is Light Energy?
The sun emits an enormous amount of solar energy. The manner in which solar energy travels can be described and measured as waves. Scientists can determine a wave’s energy amount by measuring its wavelength, the distance between two consecutive, similar points in a series of waves, such as from crest to crest or trough to trough (5.3.2). Shorter wavelengths correspond to higher energy, while longer wavelengths have lower energy.
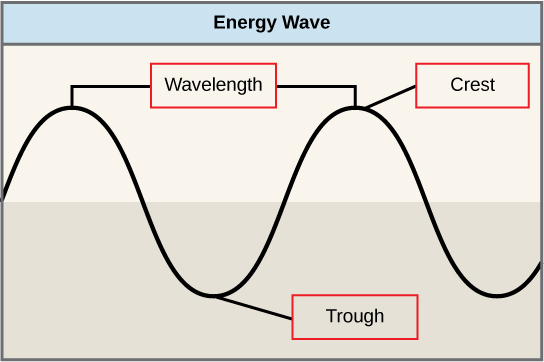
Figure 5.3.2 Description
This diagram visually represents fundamental wave properties, often used in physics and energy-related concepts such as sound, light, and water waves.
- Wavelength – Indicated as the horizontal distance between two successive crests (peaks) of the wave.
- Crest – Marked at the highest point of the wave.
- Trough – Marked at the lowest point of the wave.
The electromagnetic spectrum is the range of all possible wavelengths of radiation (Figure 5.3.3). Each wavelength corresponds to a different amount of energy carried. Visible light is a small portion of the electromagnetic spectrum that is detectable by the human eye, ranging from approximately 380 to 750 nanometers in wavelength. The visible light seen by humans as white light actually exists in a rainbow of colours. Certain objects, such as a prism or a drop of water, disperse white light to reveal these colours to the human eye – violet, blue, green, yellow, orange and red. Violet and blue have shorter wavelengths and, therefore, higher energy. At the other end of the spectrum, toward red, the wavelengths are longer and have lower energy.
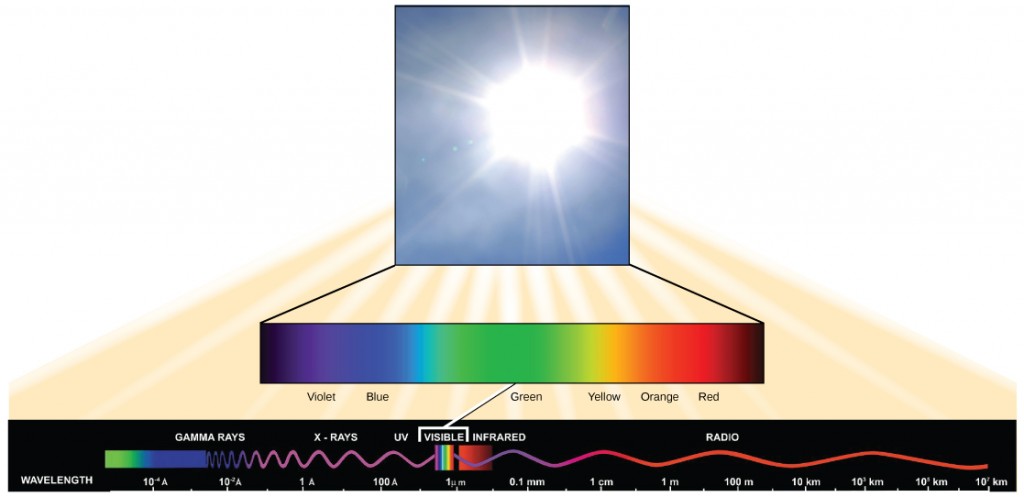
Figure 5.3.3 Description
The diagram visually explains how sunlight contains a full spectrum of electromagnetic waves, but only a small portion is visible to humans. The illustration suggests that visible light is just a tiny segment of the vast electromagnetic spectrum. The diagram consists of three main components:
- A photograph of bright sunlight in the top section, illustrating the source of visible light.
- A colour spectrum bar beneath the image of the sun, transitioning smoothly from violet to red, representing the visible spectrum of light.
- The electromagnetic spectrum at the bottom showing different types of electromagnetic waves arranged by wavelength: Gamma Rays (shortest wavelength, highest energy), X-rays, Ultraviolet (UV), Visible Light (highlighted in a small band), Infrared (IR), Radio Waves (longest wavelength, lowest energy), The wavelength scale increases from left to right, starting at 10⁻¹0 meters (angstroms) for gamma rays and extending to 10⁷ kilometers for radio waves. A small “VISIBLE” section is marked in the spectrum to show the tiny range of wavelengths that human eyes can detect.
Understanding Pigments
Light energy enters the process of photosynthesis when pigments absorb the light. A pigment is a substance that absorbs light and gives colour to plants, animals, and other materials. In plants, pigment molecules absorb only visible light for photosynthesis. Different kinds of pigments exist, each absorbing only certain wavelengths (colours) of visible light. Pigments reflect the colour of the wavelengths that they cannot absorb.
All photosynthetic organisms contain a pigment called chlorophyll, which humans see as the common green colour associated with plants. Chlorophyll a absorbs wavelengths from either end of the visible spectrum (blue and red) but not from green. Because green is reflected, chlorophyll appears green.

Other pigment types include chlorophyll b (which absorbs blue and red-orange light) and carotenoids. Each type of pigment can be identified by the specific pattern of wavelengths it absorbs from visible light, which is its absorption spectrum.
Many photosynthetic organisms have a mixture of pigments; between them, they can absorb energy from a broader range of visible-light wavelengths. Not all photosynthetic organisms have full access to sunlight. Some organisms grow underwater where light intensity decreases with depth, and the water absorbs certain wavelengths. Other organisms grow in competition for light. Plants on the rainforest floor must be able to absorb any bit of light that comes through because the taller trees block most of the sunlight (Figure 5.3.4).
Location of Light-Dependent Reactions
The light-dependent reactions occur in the thylakoid membrane of chloroplasts. Studded in the thylakoid membrane are photosystems, clusters of pigments and proteins that absorb sunlight. In the photosystem, a photon (a quantity or “packet” of light energy) is absorbed by a chlorophyll molecule and bounced around to other chlorophyll molecules until it eventually hits the chlorophyll a found in the reaction centre. The photon causes an electron in the chlorophyll to become “excited.” The energy given to the electron allows it to break free from an atom of the chlorophyll molecule. Chlorophyll is therefore said to “donate” an electron. In eukaryotes and some prokaryotes, there are two photosystems – photosystem II (PSII) and photosystem I (PSI) – which were named for the order of their discovery rather than for the order of the function.
How Light-Dependent Reactions Work
The overall purpose of the light-dependent reactions is to convert light energy into chemical energy. The chemical energy is stored by two types of energy-carrier molecules: ATP and NADPH. This chemical energy will be used by the Calvin cycle to fuel the assembly of sugar molecules.
The Light-Dependent Reactions start with PSII where a photon excites an electron in the reaction centre chlorophyll (Figure 5.3.5). This electron then passes to the next step in the light reactions, which we’ll discuss soon. For PSII to absorb more energy, that donated electron must be replaced. To do so, a water molecule is split to release the electrons, oxygen (O2) and hydrogen ions (H+). The electrons replace the donated electron from chlorophyll. The oxygen produced is a waste product that simply diffuses out of the cell and into the surrounding environment. The hydrogen ions produced play critical roles in the next step in the light-dependent reactions.
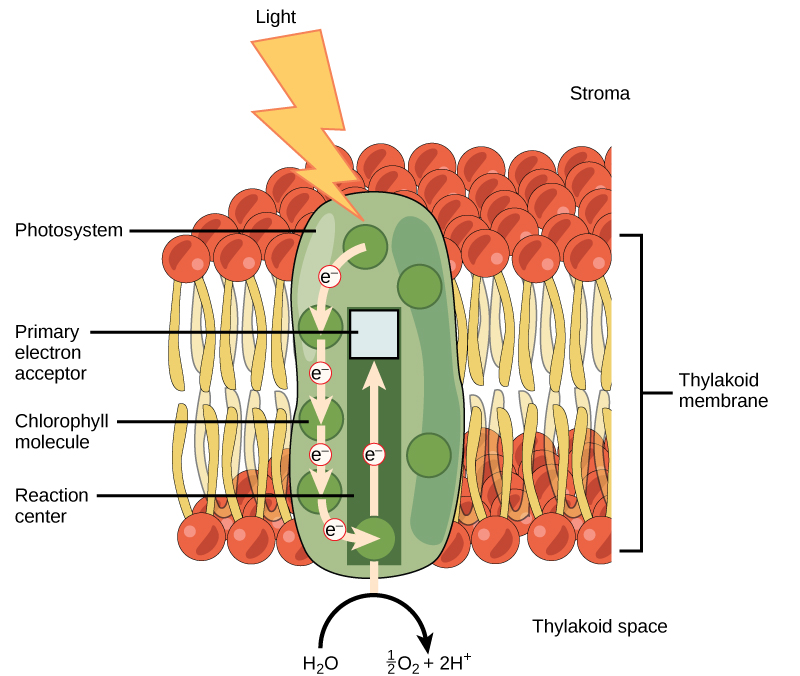
Figure 5.3.5 Description
This diagram represents the first stage of photosynthesis that occurs in the thylakoid membrane, helping convert light energy into chemical energy for use in the Calvin cycle.
- Key Components and Labels:
Light Energy (Lightning Bolt): Light energy strikes the photosystem, initiating the process of electron excitation. - Thylakoid Membrane Structure: The thylakoid membrane is shown as a bilayer composed of phospholipids (red and yellow structures).
The stroma (fluid-filled space outside the thylakoid) is labelled at the top. The thylakoid space (interior of the thylakoid) is labelled at the bottom. - Photosystem and Electron Flow: The photosystem (green, oval-shaped structure) contains chlorophyll molecules that absorb light energy.
The reaction center (bottom of the photosystem) is where electrons are excited. The primary electron acceptor (top of the photosystem) captures excited electrons. Arrows show the electron transport chain, where electrons (e⁻) move upward through different molecules.
Water Splitting (Photolysis): At the bottom, H₂O is split into oxygen (O₂) and protons (H⁺), contributing to the electron supply. Overall Process Depicted: Light energy excites electrons in the chlorophyll molecules. Electrons move through the electron transport chain. Water molecules (H₂O) split, producing oxygen (O₂) and protons (H⁺). The excited electrons continue through the electron transport chain, leading to the production of ATP and NADPH in subsequent steps.
Photosystem II transfers the excited electron to the electron transport chain (ETC). The electron transport chain is a series of proteins inside the thylakoid membrane (Figure 5.3.6). As the electron passes along these proteins, energy from the electron fuels membrane pumps that actively move hydrogen ions against their concentration gradient. After the energy is used, the electron is accepted by a pigment molecule in the next photosystem (PSI).
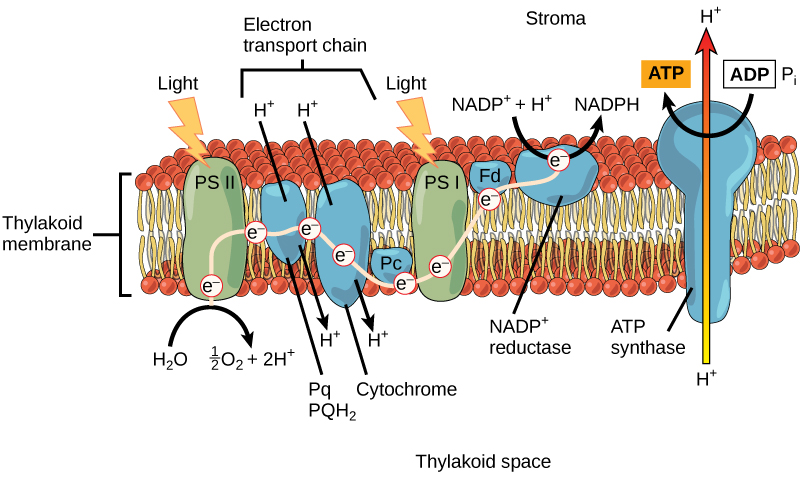
Figure 5.3.6 Description
This diagram represents the light-dependent reactions of photosynthesis, which occur in the thylakoid membrane and generate ATP and NADPH for the Calvin cycle.
Key Components and Labels:
- Thylakoid Membrane: The diagram shows the thylakoid membrane as a lipid bilayer embedded with proteins involved in the electron transport chain. The stroma (fluid-filled space outside the thylakoid) is labelled at the top. The thylakoid space (interior of the thylakoid) is labelled at the bottom.
- Photosystem II (PS II) – First Light Reaction: Light energy (lightning bolt) excites electrons in Photosystem II (PS II). Water (H₂O) is split, producing oxygen (O₂) and protons (H⁺). Excited electrons (e⁻) move to Plastoquinone (Pq/PQH₂) and then to Cytochrome complex.
- Electron Transport Chain (ETC) and Proton Gradient: As electrons move through the cytochrome complex, protons (H⁺) are pumped from the stroma into the thylakoid space, creating a proton gradient.
- Photosystem I (PS I) – Second Light Reaction: Light energy (lightning bolt) excites electrons in Photosystem I (PS I). Electrons move through Ferredoxin (Fd) to NADP⁺ reductase, reducing NADP⁺ to NADPH, which is used in the Calvin cycle.
- ATP Synthase and ATP Production: Protons (H⁺) flow down their gradient from the thylakoid space to the stroma through ATP synthase. This flow drives ADP + Pᵢ → ATP synthesis, providing energy for the Calvin cycle.
Overall Process Depicted:
Water is split, generating oxygen, protons, and electrons. Light excites electrons in PS II, starting the electron transport chain. Protons are pumped, creating a proton gradient. Electrons are re-energized in PS I and transferred to NADP⁺, forming NADPH. ATP synthase produces ATP using the proton gradient.
Generating an Energy Carrier: ATP
The electron transport chains create a higher concentration of H+ in the thylakoid space than in the stroma. This buildup of H+ has potential energy, similar to water behind a dam. The gradient causes H+ to flow back across the membrane into the stroma, where their concentration is lower. H+ pass through a channel protein and enzyme called ATP synthase. The flow of H+ through ATP synthase causes it to spin. This rotary motion generates kinetic energy, which is used to attach an inorganic phosphate (P) to a molecule of ADP, forming ATP.
Generating another Energy Carrier: NADPH
The final function of the light-dependent reactions is to generate the energy-carrier molecule NADPH from NADP+ (Nicotinamide Adenine Dinucleotide Phosphate). NADPH is an electron carrier, a molecule that transports high-energy electrons and hydrogen ions to other parts of the cell (to the Calvin cycle).
You can think of NADP⁺ as an empty Uber – ready to pick up passengers (electrons and hydrogen ions). Once it collects them, it becomes NADPH, the full Uber, which then carries the energy to its next destination.
As electrons move through the electron transport chain of the light-dependent reactions, they eventually arrive at Photosystem I. Here, they are re-energized by another photon absorbed by chlorophyll. These high-energy electrons, along with a hydrogen ion (H⁺), are then picked up by NADP⁺, converting it into NADPH.
Now that the solar energy is stored in energy carriers (ATP and NADPH), it can be used in the Calvin cycle to make a sugar molecule.
Exercises 5.3.1
Text Description
- Energize an electron
- Produce ATP
- Split a water molecule
- Synthesize glucose
- ATP
- Water
- Glucose
- Chlorophyll
- Chlorophyll
- Splitting water molecules
- The electron transport chain
- ATP synthesis
- Red and blue
- Red
- Blue
- Green
- bond
- electron
- electron
- synthase
- photon
- thylakoid
- electrochemical
- hydrogen ions
- electron transport
- NADPH
- molecule
- chlorophyll
6. Categories: Requires (goes in) and Produces (goes out)
Draggable items: ATD, ADP, NADPH, Oxygen gas, Water, Photons
Answers:
“5.2 The Light-Dependent Reactions of Photosynthesis” from Biology and the Citizen by Colleen Jones is licensed under a Creative Commons Attribution 4.0 International License, except where otherwise noted.

