5.1 Energy
Chemical reactions that take place inside living things are called biochemical reactions. The sum of all the biochemical reactions in an organism is called metabolism. Metabolism includes catabolic and anabolic chemical reactions.
Catabolic Reactions
Catabolic reactions are processes that release energy. In organisms, these reactions involve breaking down molecules into smaller units and releasing energy. An example of a catabolic reaction is the breakdown of glucose during cellular respiration, which releases the energy necessary for cells to perform vital functions.
Anabolic Reactions
Anabolic reactions are processes that absorb energy. In organisms, these reactions involve building complex molecules from simpler ones, requiring an input of energy. A prime example of an anabolic reaction is photosynthesis, where plants use energy from sunlight convert carbon dioxide and water into glucose and oxygen.
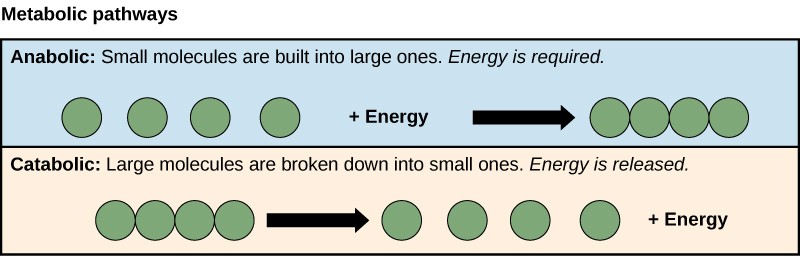
Activation Energy
All chemical reactions require energy to get started. Even exothermic reactions that release energy need a boost of energy to begin. The energy required to start a chemical reaction is called activation energy. Activation energy is like a child’s push to start going down a playground slide. The push gives the child enough energy to start moving, but once she starts, she keeps moving without being pushed again.
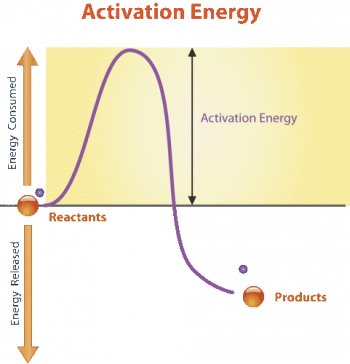
Figure 5.1.2 Description
A diagram illustrating activation energy in a chemical reaction. The graph has energy on the vertical axis and progress of response on the horizontal axis. A curved purple line represents the energy change during the reaction. It starts at a point labelled ‘Reactants,’ rises to a peak (the activation energy barrier), and then falls to a lower energy level labelled ‘Products.’ A vertical double-headed arrow labelled ‘Activation Energy’ indicates the energy required to reach the peak. The left side of the graph is labelled ‘Energy Consumed,’ and the lower portion is labelled ‘Energy Released.’
Why do chemical reactions need energy to get started? For reactions to begin, reactant molecules must bump into each other, so they must be moving, and movement requires energy. When reactant molecules bump together, they may repel each other because intermolecular forces push them apart. Energy is also needed to overcome these forces so the molecules can come together and react.
Enzymes
Most of the biochemical reactions that happen inside living organisms require help. Why is this the case? For one thing, temperatures inside living things are usually too low for biochemical reactions to occur quickly enough to maintain life. The concentrations of reactants may also be too low for them to come together and react. Where do the biochemical reactions get the help they need to proceed? From the enzymes.
An enzyme is a protein that catalyzes (speeds up) a biochemical reaction. An enzyme generally reduces the activation energy needed to start the reaction. The graph in Figure 5.1.3 shows the activation energy required for glucose to combine with oxygen. Less activation energy is required when the correct enzyme is present than when it is not.
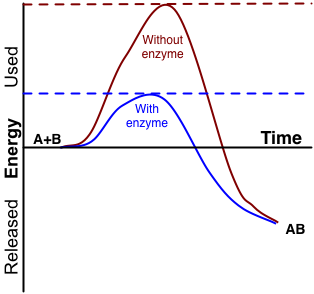
Figure 5.1.3 Description
A graph compares a chemical reaction’s activation energy with and without an enzyme. The x-axis represents time, and the y-axis represents energy, with ‘Used’ at the top and ‘Released’ at the bottom. Two curves illustrate the energy changes: a red curve labelled ‘Without enzyme’ rises to a higher peak, indicating a more significant activation energy requirement. A lower blue curve labelled ‘With enzyme’ shows a reduced activation energy requirement. The reaction starts at ‘A + B’ and ends at ‘AB.’ Dashed horizontal lines indicate the peak energy levels for each reaction path.
Enzymes function by binding to the reactant molecules and holding them in such a way as to make the chemical bond-breaking and forming processes take place more easily. It is important to remember that enzymes do not change whether a reaction is catabolic or anabolic. They only reduce the activation energy required for the response to go forward. In addition, an enzyme itself is unchanged by the reaction it catalyzes. Once one reaction has been catalyzed, the enzyme can participate in other reactions.
Enzymes are involved in most biochemical reactions and do their jobs well. A typical biochemical response that would take several days or even centuries without an enzyme will likely occur in just a split second with the proper enzyme! Without enzymes to speed up biochemical reactions, most organisms could not survive.
Enzymes are substrate-specific. The substrate of an enzyme is the specific substance it binds with. Each enzyme works only with a particular substrate, which explains why many different enzymes exist. The enzyme’s active site is located within the enzyme where the substrate binds. The active site is where the “action” happens. Since enzymes are proteins, there is a unique combination of amino acid side chains within the active site. Different properties characterize each side chain. They can be large or small, weakly acidic or basic, hydrophilic or hydrophobic, positively or negatively charged, or neutral. The unique combination of side chains creates a particular chemical environment within the active site. This specific environment is suited to bind to a certain chemical substrate.
As the enzyme and substrate come together, their interaction causes a mild shift in the enzyme’s structure, forming an ideal binding arrangement between enzyme and substrate. This dynamic binding is called induced fit and forms an enzyme-substrate complex.
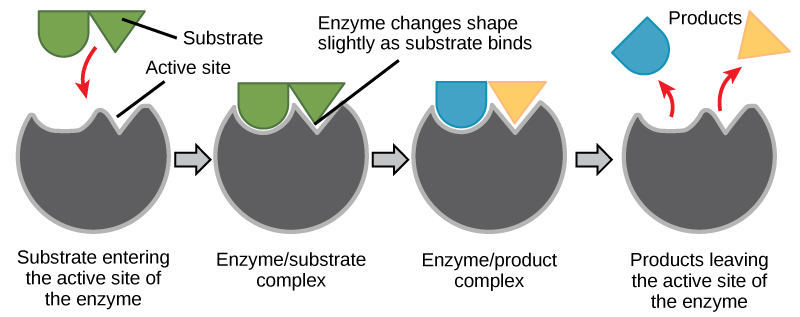
Figure 5.1.4 Description
A step-by-step diagram illustrating the enzyme-substrate interaction process. The first stage shows a substrate (a green shape) approaching the active site of an enzyme (a gray structure with an indentation). In the second stage, the substrate binds to the enzyme’s active site, forming an enzyme-substrate complex. The third stage shows the enzyme changing shape slightly as it catalyzes the reaction, resulting in an enzyme-product complex. In the final stage, the products (a blue and a yellow shape) are released from the enzyme, which remains unchanged and ready for another reaction cycle. Red arrows indicate movement throughout the process.
Enzyme Inhibitors
Enzymes can be regulated to reduce enzyme activity. An enzyme inhibitor is a molecule that binds to an enzyme and blocks its function. In competitive inhibition, an inhibitor binds to the active site and blocks the substrate from binding. In allosteric inhibition (non-competitive inhibition), an inhibitor binds to a site on the enzyme that is not the active site. This binding alters the enzyme’s overall shape, preventing the substrate from binding to the active site.
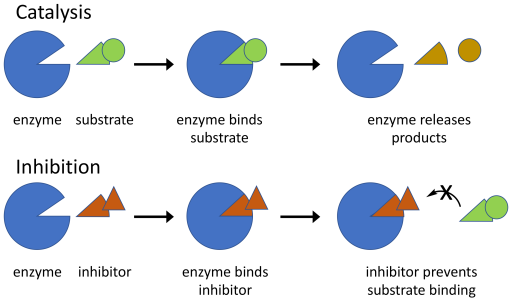
Figure 5.1.5 Description
The image illustrates two biochemical processes: Catalysis and Inhibition, using a simplified enzyme-substrate model.
- Catalysis Process: The enzyme (blue shape) is shown separately from the substrate (green shape). The substrate binds to the enzyme, fitting into its active site. The enzyme facilitates a reaction, breaking the substrate into smaller products (a yellow triangle and a yellow circle). The products are released, and the enzyme is free to catalyze another reaction.
- Inhibition Process: The enzyme is shown separately from an inhibitor (brown triangle). The inhibitor binds to the enzyme, occupying the active site. This binding prevents the substrate from attaching, blocking the enzyme’s catalytic function. This diagram visually represents how enzymes catalyze reactions and how inhibitors can regulate or block enzyme activity by preventing substrate binding.
Energy Transformations
In the scientific world, energy is the ability to do work. You can often see energy at work in living things — a bird flies through the air, a firefly glows in the dark, a dog wags its tail. Living organisms constantly use energy in less obvious ways, as well. Inside every cell of all living things, energy is needed to carry out life processes. Energy is required to break down, build up molecules, and transport many molecules across plasma membranes. All of life’s work needs energy.
There are two main categories of energy:
Kinetic Energy
Kinetic energy is the energy associated with objects in motion. For example, a moving wrecking ball, a speeding bullet, a walking person, and the rapid movement of molecules in the air all have kinetic energy.

Potential Energy
Potential energy is stored in an object due to its position or state. For instance, a motionless wrecking ball lifted two stories high has potential energy because of its position and the force of gravity acting on it. Other examples include water held behind a dam, a compressed spring, or a pulled rubber band.

A principle known as the conservation of energy explains that energy can change forms or move from place to place, but it cannot be created or destroyed. For example, light bulbs convert electrical energy into light and heat, and plants convert sunlight into chemical energy.
The principle of conservation of energy is shown in the energy transformations of a wrecking ball. Imagine a wrecking ball lifted two stories high by a crane. When the ball is lifted, energy is used to raise it against the force of gravity. This energy doesn’t disappear; instead, it is stored in the wrecking ball as potential energy due to its elevated position.
Now, if the wrecking ball is released, it starts to fall. As it falls, the potential energy is converted into kinetic energy, which is the energy of motion. When the wrecking ball reaches the ground, all the potential energy has been transformed into kinetic energy.
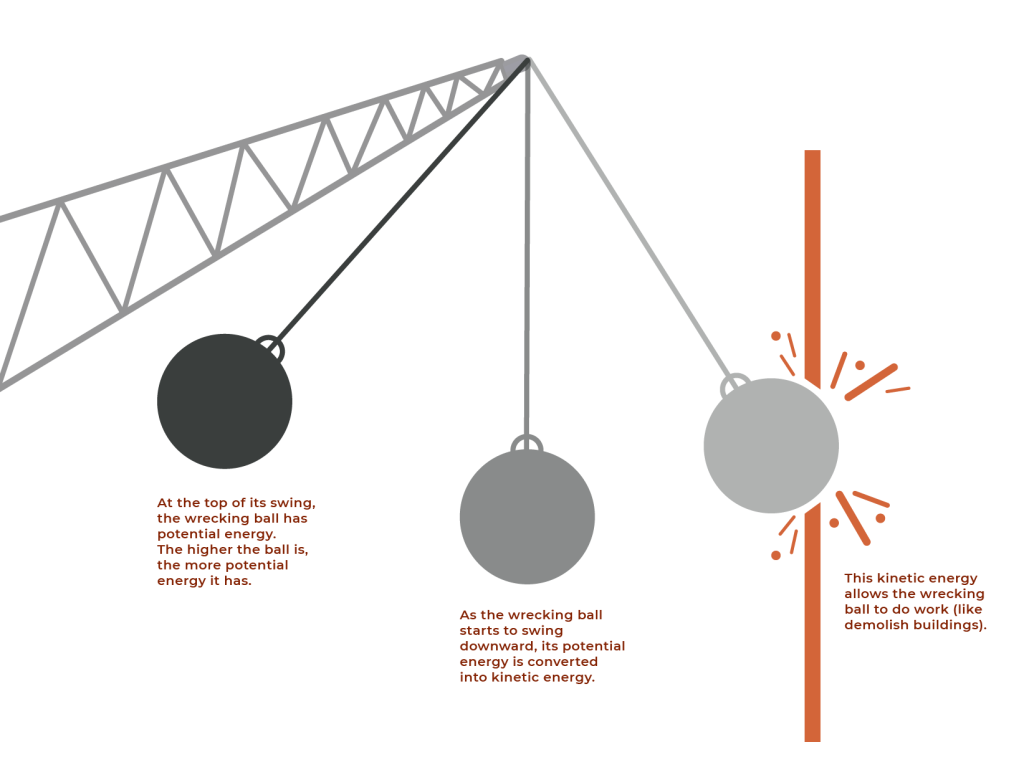
Figure 5.1.8 Description
At the top of its swing, the wrecking ball has potential energy. The higher the ball is, the more potential energy it has. As the wrecking ball starts to swing downward, its potential energy is converted into kinetic energy. This kinetic energy allows the wrecking ball to do work (like demolish buildings).
Energy in Living Organisms
Living cells use chemical energy to provide energy for various cellular processes. Chemical energy is a type of potential energy stored within the bonds of chemical compounds, such as food molecules. When these bonds are broken, the stored energy is released and can be used to perform work.
Organisms mainly use two types of molecules for chemical energy: glucose and ATP. Both molecules are used as fuels throughout the living world.
Glucose
Glucose is a simple carbohydrate with the chemical formula C6H12O6. It stores chemical energy in a concentrated, stable form. In your body, glucose is the form of energy that is carried in your blood and taken up by each of your trillions of cells. Glucose is the end product of photosynthesis and the nearly universal food for life.
ATP
ATP (adenosine triphosphate) is often referred to as the energy currency in the cell. It is the energy-carrying molecule that cells directly use to power most cellular processes (e.g., nerve impulse conduction, protein synthesis, and active transport).
ATP consists of adenosine (adenine + ribose) and a tail of three phosphate groups. Each phosphate group is negatively charged. These negative charges repel each other, which gives the tail potential energy. ATP releases energy when it gives up one of its three phosphate groups (Pi) and changes to ADP (adenosine diphosphate, which has two phosphate groups).
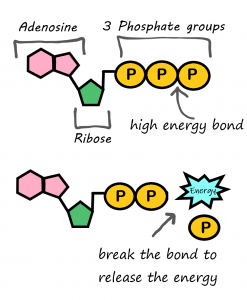
Figure 5.1.9 Description
A diagram illustrating the structure and function of ATP (adenosine triphosphate) in energy release. The top portion shows ATP with three phosphate groups (represented as yellow circles labelled ‘P’), a ribose sugar (green pentagon), and an adenosine molecule (two pink hexagons). A label indicates a high-energy bond between the last phosphate group. The bottom portion shows the ATP molecule after breaking the bond between the previous phosphate group, forming ADP (adenosine diphosphate) and releasing energy, represented by a blue burst labelled “Energy.” The diagram includes handwritten-style annotations explaining the process.
Exercise 5.1.1
Drag each item to its corresponding energy type
Text Description
Energy Types: Kinetic or Potential
Draggable items:
- A raised weight
- A stretched rubber band
- A baseball thrown by a pitcher
- An asteroid falling towards Earth
- Energy in ATP
Answers:
- Kinetic energy: A baseball thrown by a pitcher, An asteroid falling toward Earth
- Potential energy: A raised weight, A stretched rubber band, Energy in ATP
“3.10 Chemical Reactions in Living Things” from Human Biology by Christine Miller is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, except where otherwise noted.
“4.1 Energy and Metabolism” from Biology and the Citizen by Colleen Jones is licensed under a Creative Commons Attribution 4.0 International License, except where otherwise noted.

