4.4 Passive Transport
Transport Across Membranes
If a cell were a house, the plasma membrane would be the walls with windows and doors. Moving things in and out of the cell is an important function of the plasma membrane. It controls everything that enters and leaves the cell. There are two basic ways substances can cross the plasma membrane: passive transport — which requires no energy expenditure by the cell — and active transport — which requires energy from the cell.
Transport Without Energy Expenditure By The Cell
Passive transport occurs when substances cross the plasma membrane without any energy input from the cell. No energy is required because the substances move from an area with a higher concentration to an area with a lower concentration. Concentration refers to the number of particles of a substance per unit of volume. The more particles of a substance in a given volume, the higher the concentration. A substance always moves from an area where it is more concentrated to an area where it is less concentrated. This is referred to as “down the concentration gradient”.
There are several different types of passive transport, including simple diffusion, osmosis, and facilitated diffusion. Each type is described below.
Simple Diffusion
Diffusion is the movement of a substance due to a difference in concentration. It happens without any help from other molecules. The substance moves from where it is more concentrated to where it is less concentrated. Picture someone spraying perfume in the corner of a room. Do the perfume molecules stay in the corner? No, they spread out or diffuse throughout the room until they are evenly spread out. Figure 4.4.1 shows how diffusion works across a cell membrane. Substances that can squeeze between the lipid molecules in the plasma membrane by simple diffusion are generally very small, hydrophobic molecules, such as oxygen and carbon dioxide molecules.
Osmosis
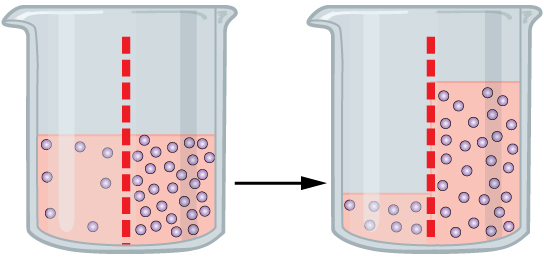
Osmosis is a special type of diffusion — the diffusion of water molecules across a membrane. Like other molecules, water moves from an area of higher concentration to an area of lower concentration. Water moves in or out of a cell until its concentration is the same on both sides of the plasma membrane. In Figure 4.4.2, the dotted red line shows a semi-permeable membrane. In the first beaker, there is an uneven concentration of solutes on either side of the membrane, but the solute cannot cross, so diffusion of the solute can’t occur. In this case, water will move to even out the concentration, as has happened on the beaker on the right side. The water levels are uneven, but the osmosis process has evened the concentration gradient.
Facilitated Diffusion
Many substances cannot simply diffuse across a membrane. Hydrophilic molecules, charged ions, and relatively large molecules (such as glucose) all need help with diffusion. This help comes from special proteins in the membrane known as transport proteins. Diffusion with the help of transport proteins is called facilitated diffusion. Several types of transport proteins exist, including channel and carrier proteins. Both are shown in Figure 4.4.3.
Channel proteins form pores (or tiny holes) in the membrane. This allows water molecules and small ions to pass through the membrane without contacting the lipid molecules’ hydrophobic tails in the membrane’s interior.
Carrier proteins bind with specific ions or molecules. In doing so, they change shape. Carrier proteins carry the ions or molecules across the membrane as they change shape.
Transport and Homeostasis
For a cell to function normally, the inside of it must maintain a stable state. The concentrations of salts, nutrients, and other substances must be kept within certain ranges. The state in which stable conditions are maintained inside a cell (or an entire organism) is called homeostasis. Homeostasis requires constant adjustments because conditions always change inside and outside the cell. As described in this section, the transport of substances into and out of cells plays an important role in homeostasis. By allowing the movement of substances into and out of cells, transport keeps conditions within normal ranges inside the cells and throughout the organism as a whole.
Exercises 4.4.1
Text Description
- Plasma membrane
- Vesicle
- Golgi apparatus
- Nucleus
- No, it must have been hypotonic, as a hypotonic solution would cause water to enter the cells, thereby making them burst.
- Yes, it must have been isotonic, as an isotonic solution would cause water to enter the cells, thereby making them burst.
- Water moves through a semipermeable membrane in osmosis because there is a concentration gradient across the membrane of solute and solvent. The solute cannot effectively move to balance the concentration on both sides of the membrane, so water moves to achieve this balance.
- Water moves through a permeable membrane in osmosis because there is a balanced concentration gradient across the membrane of solute and solvent. The solute has moved to balance the concentration on both sides of the membrane to achieve this balance.
- Water moves from an area with a low concentration of solutes to an area with a higher one
- Water moves throughout the cytoplasm
- Water moves from an area with a high concentration of other solutes to a lower one
- Water moves from an area with a high concentration of other solutes to a lower one
- particle size
- concentration gradient
- membrane surface area
- temperature
Answers:
- False
- True
- False
- Plasma membrane
- No, it must have been hypotonic, as a hypotonic solution would cause water to enter the cells, thereby making them burst.
- Water moves through a semipermeable membrane in osmosis because there is a concentration gradient across the membrane of solute and solvent. The solute cannot effectively move to balance the concentration on both sides of the membrane, so water moves to achieve this balance.
- Water moves from an area with a low concentration of solutes to an area with a higher one.
- Concentration gradient.
Tonicity
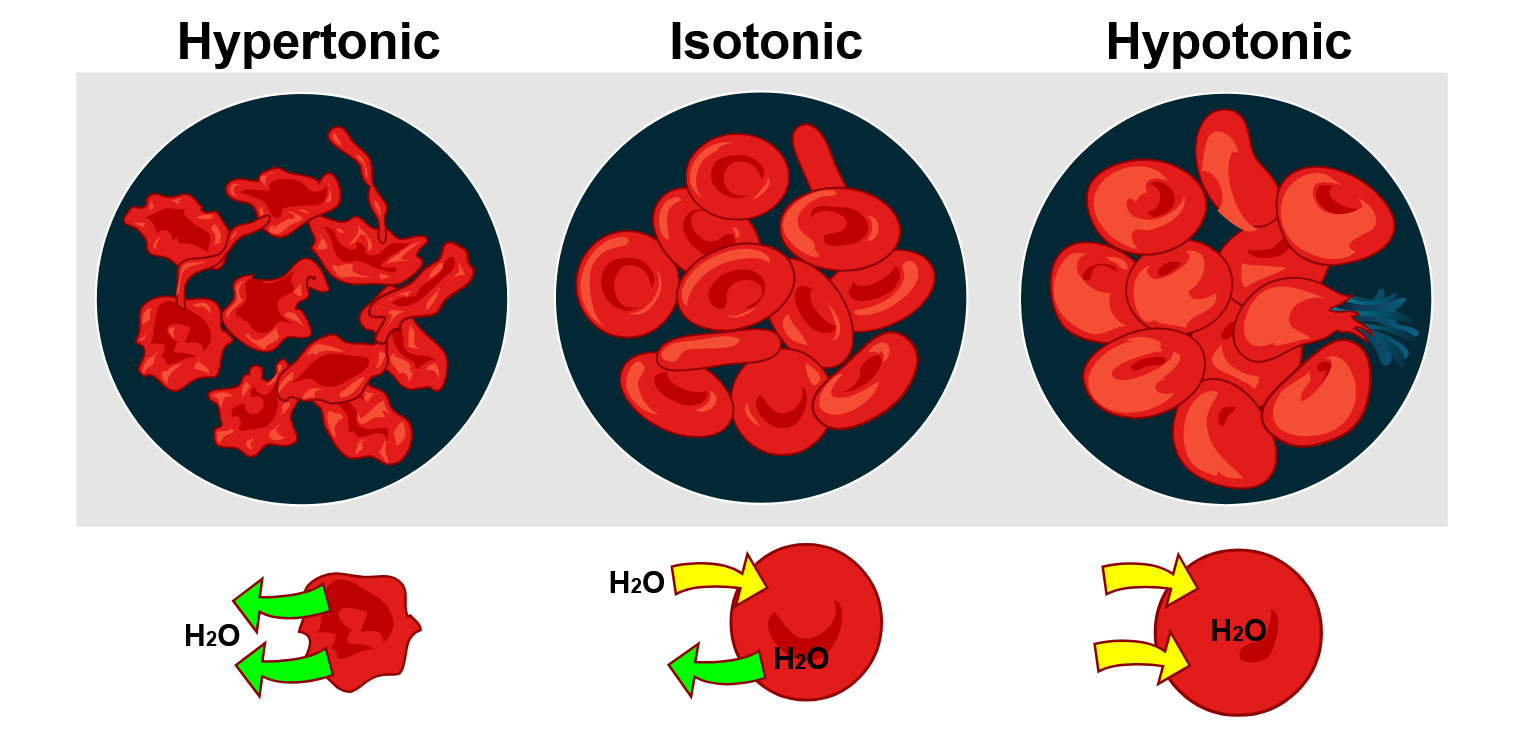
Tonicity describes the concentration of solutes in a solution outside the cell compared to inside the cell. Different tonicities affect the cell’s volume and pressure. There are three types of tonicity: hypotonic, isotonic, and hypertonic.
In a hypotonic solution, such as tap water, the extracellular fluid has a lower concentration of solutes than the fluid inside the cell, and water enters the cell. (In living systems, the point of reference is always the cytoplasm, so the prefix hypo– means that the extracellular fluid has a lower concentration of solutes than the cell cytoplasm.) It also means that the extracellular fluid has a higher water concentration than the cell. Water will follow its concentration gradient and enter the cell in this situation. This may cause an animal cell to burst or lyse.
In a hypertonic solution (the prefix hyper– refers to the extracellular fluid having a higher concentration of solutes than the cell’s cytoplasm), the fluid contains less water than the cell does, such as seawater. Because the cell has a lower concentration of solutes, the water will leave the cell. In effect, the solute is drawing the water out of the cell. This may cause an animal cell to shrivel or crenate.
In an isotonic solution, the extracellular fluid has the same osmolarity as the cell. If the concentration of solutes in the cell matches that of the extracellular fluid, there will be no net movement of water into or out of the cell. In hypertonic, isotonic, and hypotonic solutions, blood cells take on characteristic appearances (Figure 4.4.4).
A doctor injects a patient with what the doctor thinks is an isotonic saline solution. The patient dies, and an autopsy reveals that many red blood cells have been destroyed. Do you think the solution the doctor injected was really isotonic?
(Answer: No, it must have been hypotonic, as a hypotonic solution would cause water to enter the cells, thereby making them burst.)
Some organisms, such as plants, fungi, bacteria, and protists, have cell walls surrounding the plasma membrane and preventing cell lysis. The plasma membrane can only expand to the limit of the cell wall, so the cell will not lyse. In fact, the cytoplasm in plants is always slightly hypertonic compared to the cellular environment, and water will always enter a cell if water is available. This influx of water produces turgor pressure, which stiffens the cell walls of the plant (Figure 4.4.5). In nonwoody plants, turgor pressure supports the plant. If the plant cells become hypertonic, as occurs in drought, or if a plant is not watered adequately, water will leave the cell. Plants lose turgor pressure in this condition and wilt.
“4.7 Passive Transport” from Human Biology by Christine Miller is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, except where otherwise noted.
“3.5 Passive Transport” from Biology and the Citizen by Colleen Jones is licensed under a Creative Commons Attribution 4.0 International License, except where otherwise noted.

