3.4 Proteins
Proteins are one of the most abundant organic molecules in living systems and have the most diverse range of functions of all macromolecules. Each cell in a living system may contain thousands of different proteins, each with a unique function. Their structures, like their functions, vary greatly. However, they are all polymers of amino acids arranged in a linear sequence and connected by covalent bonds.
Amino acids are the monomers that make up proteins (Figure 3.4.1). Each amino acid has the same fundamental structure, which consists of a central carbon atom bonded to an amino group (NH2), a carboxyl group (COOH), and a hydrogen atom. Every amino acid also has another atom or group of atoms bonded to the central atom, known as the side chain or R group. There are 20 common amino acids commonly found in proteins, each with a different R group that determines its chemical nature (whether it is acidic, basic, polar, or nonpolar).
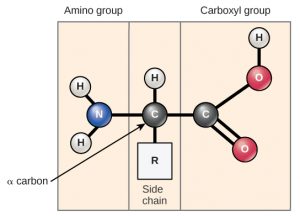
The sequence and number of amino acids ultimately determine a protein’s shape, size, and function. Each amino acid is attached to another amino acid by a covalent bond, a peptide bond formed by a dehydration reaction. The carboxyl group of one amino acid and the amino group of a second amino acid combine, releasing a water molecule. The resulting bond is the peptide bond.
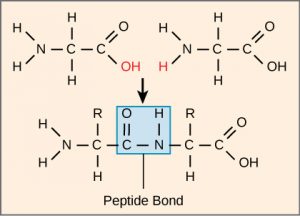
The products formed by such a linkage are called polypeptides. While the terms polypeptide and protein are sometimes used interchangeably, a polypeptide is technically a polymer of amino acids. In contrast, the term protein is used for a polypeptide that has folded into a distinct shape and has a specific function.
Protein Function
The functions of proteins can be very diverse because the function depends on the protein’s shape. The order of the amino acids determines the shape of a protein. Proteins are often hundreds of amino acids long and can have very complex shapes because there are so many possible orders for the 20 amino acids.
| Type | Examples | Functions |
|---|---|---|
| Enzymes | Digestive enzymes (amylase, lipase) | Help catalyze chemical reactions |
| Transport | Hemoglobin | Carry substances throughout the body |
| Structural | Hair (keratin), ligaments (collagen) | Construct and provide support for different structures |
| Hormones | Insulin, adrenaline | Coordinate the activity of different body systems |
| Defence | Antibodies, immunoglobulins | Protect the body from foreign pathogens |
| Contractile | Actin, myosin | Allow muscle contraction |
| Storage | Legume storage proteins, egg white (albumin) | Provide nourishment in the early development of the embryo and the seedling |
Protein Structure
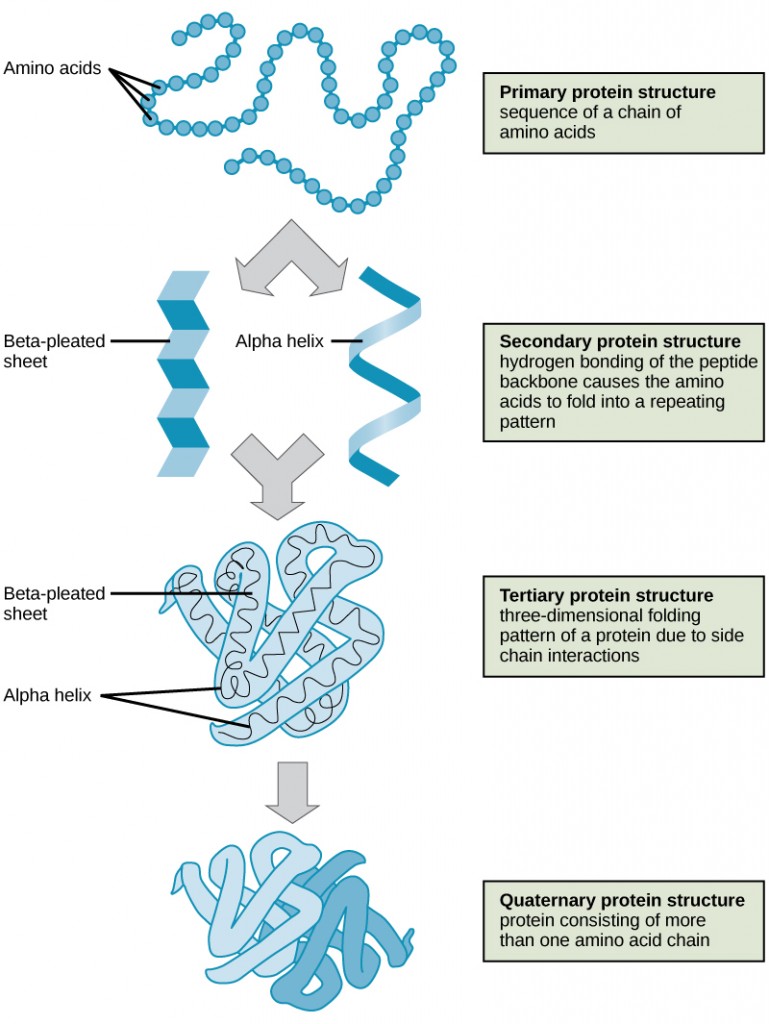
The shape of a protein is critical to its function. To understand how the protein gets its final shape or conformation, we need to understand the four levels of protein structure: primary, secondary, tertiary, and quaternary.
Primary
Primary structure is the unique sequence and number of amino acids in a polypeptide chain. The unique sequence for every protein is ultimately determined by the gene that encodes the protein. Any change in the gene sequence may lead to a different amino acid being added to the polypeptide chain, causing a change in protein structure and function.
A hemoglobin molecule is made up of four chains, each with about 150 amino acids, totalling around 600 amino acids. Sickle cell anemia is a genetic disorder that results in a change in just one of those 600 amino acids. That one change causes the normally biconcave, or disc-shaped, red blood cells to assume a crescent or “sickle” shape, which clogs arteries. This can lead to a variety of serious health problems, such as breathlessness, dizziness, headaches, and abdominal pain, and dramatically decrease life expectancy in the affected individuals.
Secondary
Secondary structure refers to the localized folding patterns resulting from interactions between the non-R group portions of amino acids. The alpha (α)-helix and beta (β)-pleated sheet structures are the most common. Both structures are held in shape by hydrogen bonds.
Tertiary
Tertiary structure is the unique three-dimensional structure of a polypeptide. This structure is primarily caused by chemical interactions between various R groups. There may be ionic bonds or hydrogen bonds formed between R groups on different amino acids. Additionally, during protein folding, the hydrophobic R groups of nonpolar amino acids are positioned inside the protein, while the hydrophilic R groups are outside.
Quaternary
Quaternary structure involves the arrangement of multiple polypeptide chains which interact to create a functional protein. For example, as mentioned, hemoglobin is a combination of four polypeptide chains.

Each protein has a unique sequence and shape held together by chemical interactions. When exposed to changes in temperature, pH, or chemicals, the protein may lose its shape, a process known as denaturation. Denaturation is often reversible because the primary structure remains intact, so the protein can regain its function once the denaturing agent is removed. Sometimes denaturation is irreversible, resulting in a loss of function. An example of protein denaturation is seen when an egg is fried or boiled; the albumin protein in the liquid egg white denatures in the heat, changing from clear to opaque white.
“Proteins” from Principles of Biology by Catherine Creech is licensed under a Creative Commons Attribution 4.0 International License, except where otherwise noted.
“2.3 Biological Molecules” from Biology and the Citizen by Colleen Jones is licensed under a Creative Commons Attribution 4.0 International License, except where otherwise noted.

