3.1 Organic Compounds
Food provides an organism with nutrients—the matter it needs to survive. Many of these critical nutrients come from biological macromolecules, or large molecules necessary for life. These macromolecules are built from different combinations of smaller organic molecules. What specific types of biological macromolecules do living things require? How are these molecules formed? What functions do they serve? In this chapter, we will explore these questions.


Biological molecules are made up of subunits connected together by covalent bonds. Covalent bonds are strong and can hold these subunits together into long chains. If hydrogen bonds connected them, the subunits would easily separate from each other, and the biological molecule would come apart. If ionic bonds connected the subunits, the biological molecule would likely fall apart if it came into contact with water. Each type of biological molecule is made up of different subunits.
There are four major classes of biological macromolecules (carbohydrates, lipids, proteins, and nucleic acids), and each is an important component of the cell and performs a wide array of functions. Combined, these molecules make up the majority of a cell’s mass. Biological macromolecules are organic, meaning that they contain carbon atoms. In addition, they may contain atoms of hydrogen, oxygen, nitrogen, phosphorus, sulphur, and additional minor elements.
Carbon
It is often said that life is “carbon-based.” This means that carbon atoms, bonded to other carbon atoms or other elements, form the fundamental components of many, if not most, of the molecules found uniquely in living things. Other elements play important roles in biological molecules, but carbon certainly qualifies as the “foundation” element for molecules in living things. It is the versatile bonding properties of carbon atoms that play an important role.
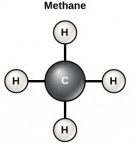
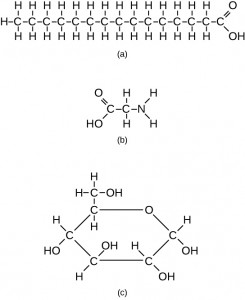
Carbon contains four electrons in its outer shell. Therefore, it can form four covalent bonds with other atoms or molecules. The simplest organic carbon molecule is methane (CH4), in which four hydrogen atoms bind to a carbon atom (Figure 3.1.3). However, carbon atoms can also bond to other carbon atoms to make long chains, often called carbon skeletons (Figure 3.1.4a). These skeletons form the backbone of organic molecules and can vary in length, branching, and the presence of double or triple bonds. Other elements, such as nitrogen, oxygen, and phosphorus, often branch off of the carbon skeleton (Figure 3.2.1b). These skeletons can also form rings (Figure 3.1.4c). This diversity of molecular forms accounts for the diversity of functions of biological macromolecules. It is based to a large degree on the ability of carbon to form multiple bonds with itself and other atoms.
Dehydration Synthesis
Most macromolecules are made from single subunits, or building blocks, called monomers. The monomers combine with each other using covalent bonds to form larger molecules known as polymers. In doing so, monomers release water molecules as byproducts. This type of reaction is known as dehydration synthesis, which means “to put together while losing water.”
In a dehydration synthesis reaction (Figure 3.1.5a), the hydrogen of one monomer combines with the hydroxyl (OH) group of another monomer, releasing a molecule of water. At the same time, the monomers share electrons and form covalent bonds. As additional monomers join, this chain of repeating monomers forms a polymer. Different types of monomers can combine in many configurations, giving rise to a diverse group of macromolecules. Even one kind of monomer can combine in various ways to form several different polymers. For example, glucose monomers are the constituents of starch, glycogen, and cellulose.
Hydrolysis
Polymers are broken down into monomers in a process known as hydrolysis, which means “to split water,” a reaction in which a water molecule is used during the breakdown (Figure 3.1.5b). During these reactions, the polymer is broken into two components: one part gains a hydrogen atom (H+), and the other gains a hydroxyl molecule (OH–) from a split water molecule.
Dehydration and hydrolysis reactions are catalyzed, or “sped up,” by specific enzymes. Dehydration reactions involve the formation of new bonds, requiring energy, while hydrolysis reactions break bonds and release energy. Most macromolecules have similar reactions, but each monomer and polymer reaction is specific for its group. For example, in our bodies, food is broken down into smaller molecules by enzymes in the digestive system. This allows for easy absorption of nutrients by cells in the intestine. A specific enzyme breaks down each macromolecule. For instance, carbohydrates are broken down by amylase, sucrase, lactase, or maltase. Proteins are broken down by pepsin, peptidase, and hydrochloric acid. Lipids are broken down by lipases. The breakdown of these macromolecules provides energy for cellular activities and the components that build new molecules.
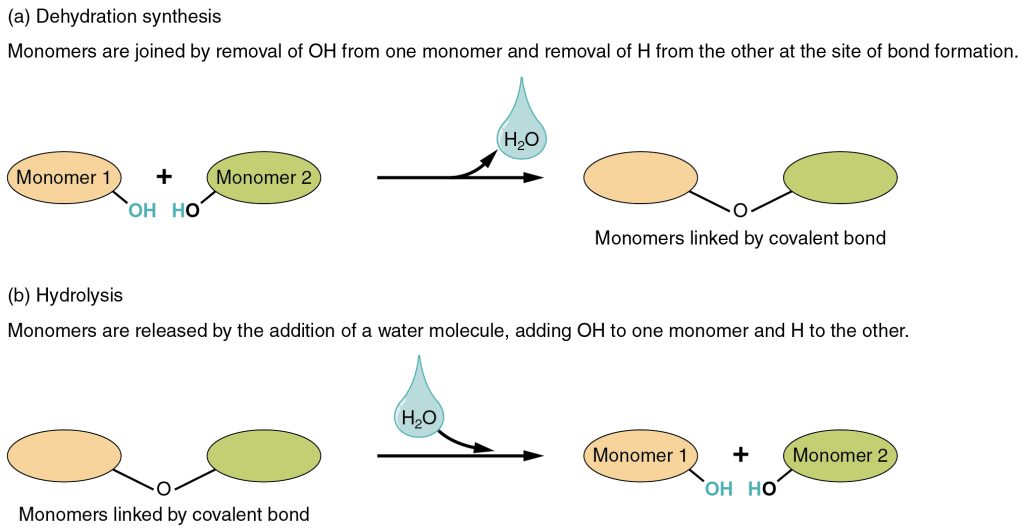
Image Description
a. Dehydration Synthesis
Monomers are joined by the removal of OH from one monomer and the removal of H from the other at the site of bond formation. Water is removed, and monomers are linked by a covalent bond.
b. Hydrolysis
Monomers are released by the addition of a water molecule, adding OH to one monomer and H to the other, resulting in monomers linked by a covalent bond into two monomers.
Exercise 3.1.1
Text Description
Drag the words into the correct boxes
- monomers
- organic
- biological macromolecules
- covalent
Answers:
Carbohydrates, lipids, proteins, and nucleic acids are classes of biological macromolecules. These molecules are organic, which means they contain carbon. Subunits of these molecules are called monomers and are connected together by strong covalent bonds.
“Chapter 2: Introduction to the Chemistry of Life” from Biology and the Citizen by Colleen Jones is licensed under a Creative Commons Attribution 4.0 International License, except where otherwise noted.
“Biological Molecules” from Principles of Biology by Catherine Creech is licensed under a Creative Commons Attribution 4.0 International License, except where otherwise noted.
“2.3 Biological Molecules” from Biology and the Citizen by Colleen Jones is licensed under a Creative Commons Attribution 4.0 International License, except where otherwise noted.
“Foundations of Biology 2.0 [PDF] by Shannon Compton, Amanda Murray Hyde, and Jennifer Williams is licensed under a CC BY 4.0, except where otherwise noted.

