2.1 Atoms and Elements
At its most fundamental level, life is made up of matter. Matter occupies space and has mass. All matter comprises elements, substances that cannot be broken down or transformed chemically into other substances. Each element is made of atoms, each with a constant number of protons and unique properties. 118 elements have been defined; however, only 94 occur naturally, and fewer than 30 are found in living cells. Therefore, the remaining 26 elements are unstable, have not existed for long, are theoretical, and have yet to be detected.
Each element is designated by its chemical symbol (H, N, O, C, and Na) and possesses unique properties. These properties allow elements to combine and bond with each other in specific ways. Two or more elements that combine in a fixed ratio are called compounds. Compounds are more common than pure elements. An example of a compound is table salt or sodium chloride, which has equal parts of sodium (Na) and chlorine (Cl).
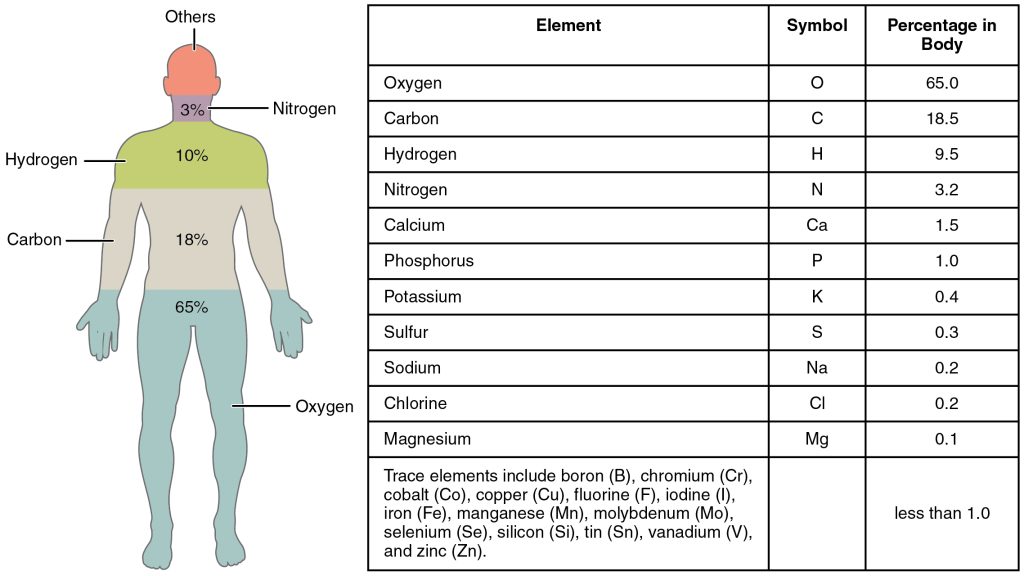
The four elements common to all living organisms are oxygen (O), carbon (C), hydrogen (H), and nitrogen (N).
Atoms
An atom is the smallest unit of matter that retains all of the chemical properties of an element. For example, one hydrogen atom has all of the properties of the element hydrogen, such as it exists as a gas at room temperature and bonds with oxygen to create a water molecule. Hydrogen atoms cannot be broken down into anything smaller while still retaining the properties of hydrogen. If a hydrogen atom were broken down into subatomic particles, it would no longer have the properties of hydrogen.
All atoms contain protons, electrons, and neutrons (Figure 2.1.2). The only exception is hydrogen (H), which is made of protons and one electron. A proton is a positively charged particle that resides in the nucleus (the core of the atom) of an atom and has a mass of 1 and a charge of +1. An electron is a negatively charged particle that travels in the space around the nucleus. In other words, it resides outside of the nucleus. It has a negligible mass and has a charge of –1.
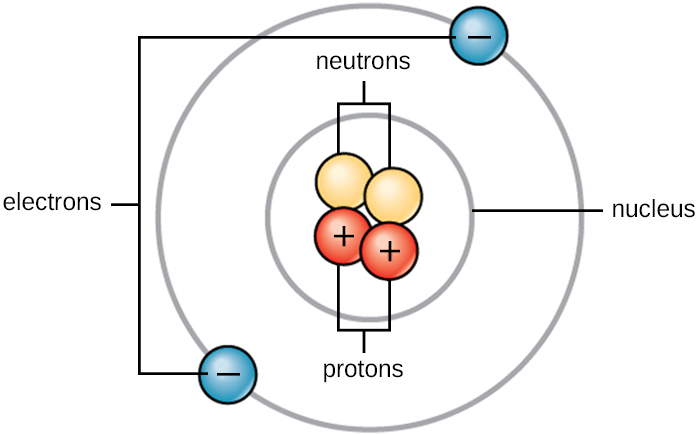
Neutrons, like protons, reside in the nucleus of an atom. They have a mass of 1 and no charge. The positive (protons) and negative (electrons) charges balance each other in a neutral atom with a net zero charge.
Because protons and neutrons each have a mass of 1, the mass of an atom is equal to the number of protons and neutrons of that atom. The number of electrons does not factor into the overall mass because their mass is so tiny.
Exercise 2.1.1
Text Description
- _____ Positively charged particle that resides in the nucleus.
- _____ The smallest component of an element that retains all of the chemical properties of that element.
- _____ Negatively charged particle found outside the nucleus.
- _____ Particle in the nucleus without a charge.
- electron
- atom
- neutron
- proton
Answers:
- proton: Positively charged particle that resides in the nucleus.
- atom: The smallest component of an element that retains all of the chemical properties of that element.
- electron: Negatively charged particle found outside the nucleus.
- neutron: Particle in the nucleus without a charge.
At the most basic level, all organisms are made of a combination of elements. An element is a substance whose atoms all have the same number of protons. They contain atoms that combine to form molecules. In multicellular organisms, such as animals, molecules can interact to create cells that combine to form tissues, which make up organs. These combinations continue until entire multicellular organisms are formed.
Each element has its unique properties. Each contains a different number of protons and neutrons, giving it its atomic and mass numbers. An element’s atomic number equals the number of protons that element contains. The mass number, or nuclear mass, is the number of protons and neutrons of that element. Therefore, it is possible to determine the number of neutrons by subtracting the atomic number from the mass number.
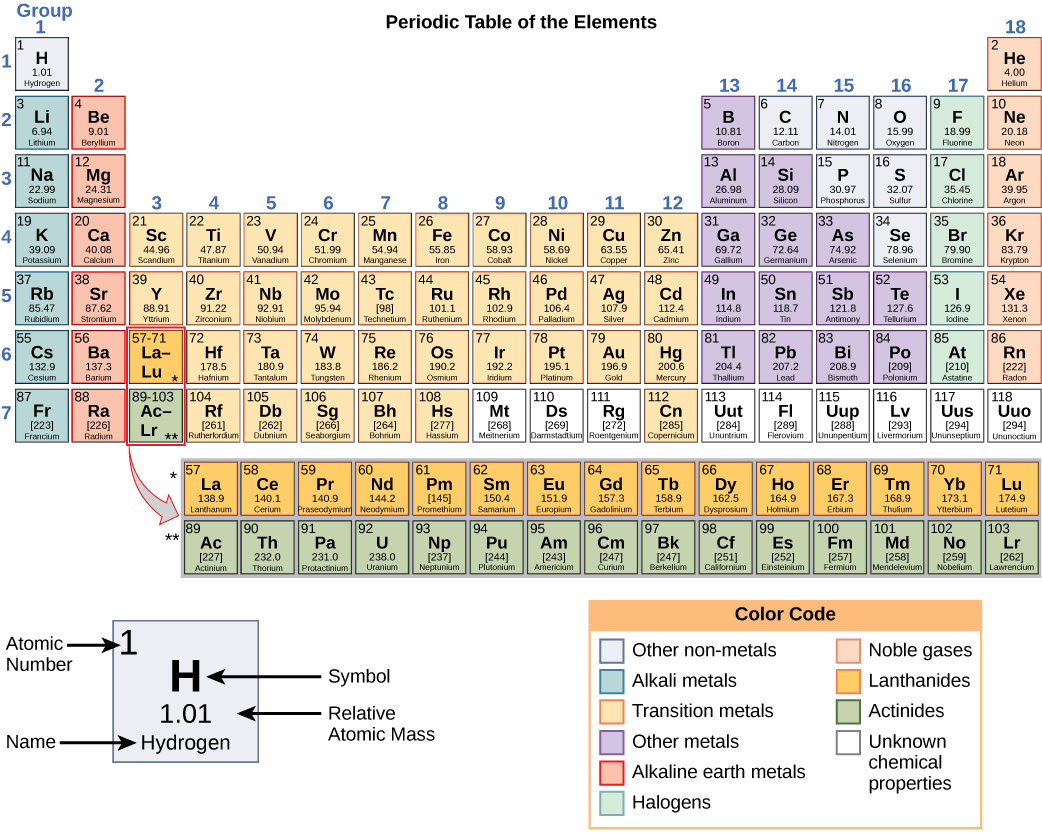
These numbers provide information about the elements and how they react when combined. Different elements have different melting and boiling points and are in various states (liquid, solid, or gas) at room temperature. They also combine in different ways. Some form specific types of bonds, whereas others do not. How they combine is based on the number of electrons present. Because of these characteristics, the elements are arranged into the periodic table of elements, a chart of the elements that includes the atomic number and relative atomic mass of each component. The periodic table also provides key information about the properties of elements (Figure 2.1.3) —often indicated by colour-coding. The arrangement of the table also shows how the electrons in each element are organized and provides essential details about how atoms will react with each other to form molecules.
Isotopes are different forms of the same element with the same number of protons but a different number of neutrons. Some elements, such as carbon, potassium, and uranium, have naturally occurring isotopes. Carbon-12, the most common carbon isotope, contains six protons and six neutrons. Therefore, it has a mass number of 12 (six protons and six neutrons) and an atomic number of 6 (which makes it carbon). Carbon-14 contains six protons and eight neutrons. Therefore, it has a mass number of 14 (six protons and eight neutrons) and an atomic number of 6, meaning it is still the element carbon. These two alternate forms of carbon are isotopes. Some isotopes are unstable and will lose protons, other subatomic particles, or energy to form more stable elements. These are called radioactive isotopes or radioisotopes.
“2.1 The Building Blocks of Molecules” from Biology and the Citizen by Colleen Jones is licensed under a Creative Commons Attribution 4.0 International License, except where otherwise noted.
“Atoms” from Principles of Biology by Catherine Creech is licensed under a Creative Commons Attribution 4.0 International License, except where otherwise noted.

