3.3 Lipids

Lipids include a diverse group of compounds that are united by a common feature. Lipids are hydrophobic (“water-fearing”) or insoluble in water because they are nonpolar molecules. This is because they are hydrocarbons that include only nonpolar carbon-carbon or carbon-hydrogen bonds. Lipids perform many different functions in a cell. Cells store energy for long-term use in the form of lipids called fats. Lipids also provide insulation from the environment for plants and animals. For example, they help keep aquatic birds and mammals dry because of their water-repelling nature. Lipids are also the building blocks of many hormones and are an important constituent of the plasma membrane. Lipids include fats, phospholipids, and steroids.
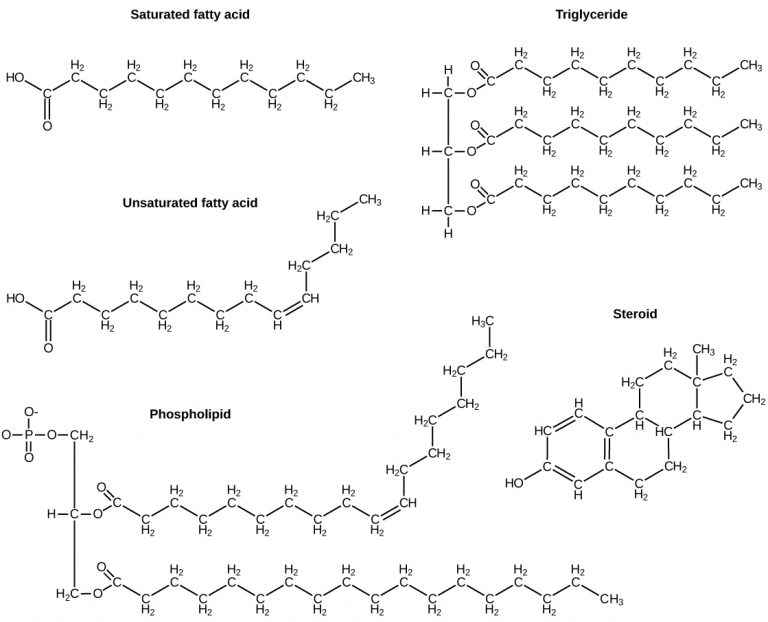
Fats
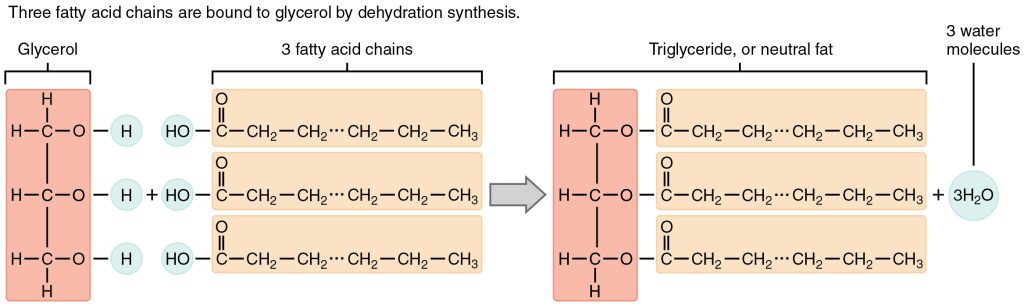
A fat molecule, such as a triglyceride, consists of two main components—glycerol and fatty acids. Glycerol is an organic compound with three carbon atoms, five hydrogen atoms, and three hydroxyl (–OH) groups. Fatty acids have a long chain of hydrocarbons to which an acidic carboxyl group is attached, hence the name “fatty acid.” The number of carbons in the fatty acid may range from 4 to 36; the most common are those containing 12–18 carbons. In a fat molecule, a fatty acid is attached to each of the three oxygen atoms in the –OH groups of the glycerol molecule with a covalent bond.
During this covalent bond formation, three water molecules are released. The three fatty acids in the fat may be similar or dissimilar. These fats are also called triglycerides because they have three fatty acids.
Fatty acids may be saturated or unsaturated. In a fatty acid chain, the fatty acid is saturated if there are only single bonds between neighbouring carbons in the hydrocarbon chain. Saturated fatty acids are saturated with hydrogen; in other words, the number of hydrogen atoms attached to the carbon skeleton is maximized.
When the hydrocarbon chain contains a double bond, the fatty acid is an unsaturated fatty acid.
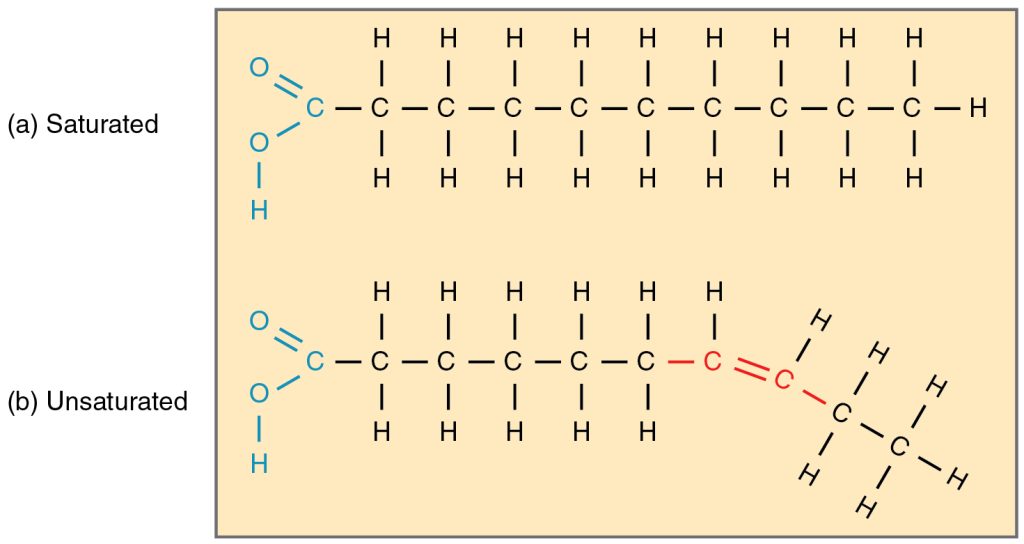
Most unsaturated fats are liquid at room temperature and are called oils. If there is one double bond in the molecule, it is known as a monounsaturated fat (e.g., olive oil), and if there is more than one double bond, it is known as a polyunsaturated fat (e.g., canola oil).
Saturated fats tend to get packed tightly and are solid at room temperature. Examples of saturated fats are animal fats with stearic acid and palmitic acid contained in meat and fat with butyric acid contained in butter. Mammals store fats in specialized cells called adipocytes, where fat globules occupy most of the cell. In plants, fat or oil is stored in seeds and used as an energy source during embryonic development.
Oils are usually of plant origin and contain unsaturated fatty acids. The double bond causes a bend or a “kink” that prevents the fatty acids from packing tightly, keeping them liquid at room temperature. Olive oil, corn oil, canola oil, and cod liver oil are examples of unsaturated fats. Unsaturated fats help to improve blood cholesterol levels, whereas saturated fats contribute to plaque formation in the arteries, which increases the risk of a heart attack.
Like carbohydrates, fats have received a lot of bad publicity. It is true that eating an excess of fried foods and other “fatty” foods leads to weight gain. However, fats do have important functions. Fats serve as long-term energy storage. They also provide insulation for the body and protect vital organs. Therefore, “healthy” unsaturated fats in moderate amounts should be consumed on a regular basis.
Phospholipids
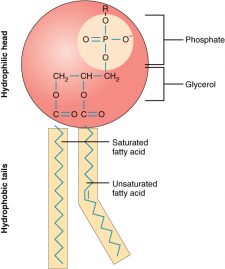
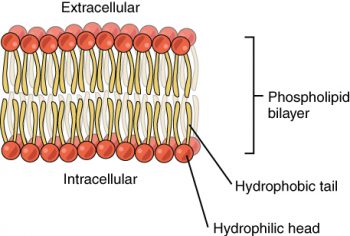
Phospholipids are a major component of the cell membranes of all living things. Each phospholipid molecule has a “tail” consisting of two long fatty acids and a “head” consisting of a phosphate group and glycerol molecule (see Figure 3.3.5). The phosphate group is a small, negatively charged molecule, causing it to be hydrophilic or attracted to water. The fatty acid tail of the phospholipid is hydrophobic or repelled by water. These properties allow phospholipids to form a two-layered cell membrane called a bilayer.
As shown in Figure 3.3.6, a phospholipid bilayer forms when many phospholipid molecules line up tail to tail, forming an inner and outer surface of hydrophilic heads. The hydrophilic heads point toward the cell’s watery extracellular space and the watery inside space (lumen). The hydrophobic fatty acids are nestled in the inner space of the bilayer.
Steroids
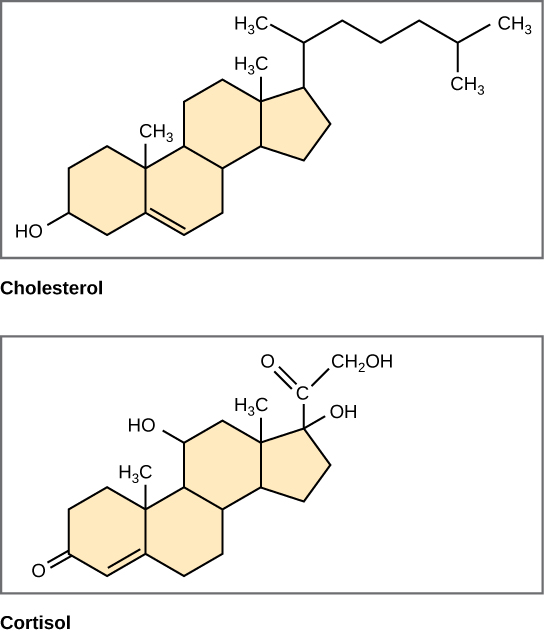
Steroids are lipids with a ring structure. Each steroid has a core of four fused carbon rings (pictured in Figure 3.5.7). Steroids vary by the other components attached to this four-ring core. Hundreds of steroids are found in plants, animals, and fungi, but most steroids have one of just two principal biological functions. Some steroids (such as cholesterol) are important components of cell membranes, while many other steroids are hormones, which are messenger molecules. In humans, steroid hormones include cortisol — a fight-or-flight hormone — and the sex hormones estrogen, progesterone, and testosterone.
Exercise 3.3.1
Text Description
- Both fats and oils
- None of the above
- Oils
- Fats
- Steroids
- Fats
- Oils
- Phospholipids
- Fats and cholesterol
- Cholesterol and phospholipids
- Fats and phospholipids
- Cholesterol and oils
“2.3 Biological Molecules” from Biology and the Citizen by Colleen Jones is licensed under a Creative Commons Attribution 4.0 International License, except where otherwise noted.

