3.2 Carbohydrates
Carbohydrates are macromolecules with which most consumers are somewhat familiar. To lose weight, some individuals adhere to “low-carb” diets. Athletes, in contrast, often “carb-load” before important competitions to ensure that they have sufficient energy to compete at a high level. Carbohydrates are, in fact, an essential part of our diet; grains, fruits, and vegetables are all natural sources of carbohydrates. Carbohydrates provide energy to the body, particularly through glucose, a simple sugar. Carbohydrates also have other important functions in humans, animals, and plants.
Carbohydrates can be represented by the formula (CH2O)n, where n is the number of carbon atoms in the molecule. In other words, the carbon to hydrogen to oxygen ratio is 1:2:1 in carbohydrate molecules. Carbohydrates are classified into three subtypes: monosaccharides, disaccharides, and polysaccharides.
Monosaccharides
Monosaccharides (mono- = “one”; sacchar- = “sweet”) are simple sugars, the most common of which is glucose. In monosaccharides, carbon atoms usually range from three to six. Most monosaccharide names end with the suffix -ose. Monosaccharides may exist as linear chains or ring-shaped molecules; in aqueous solutions, they are usually found in the ring form.
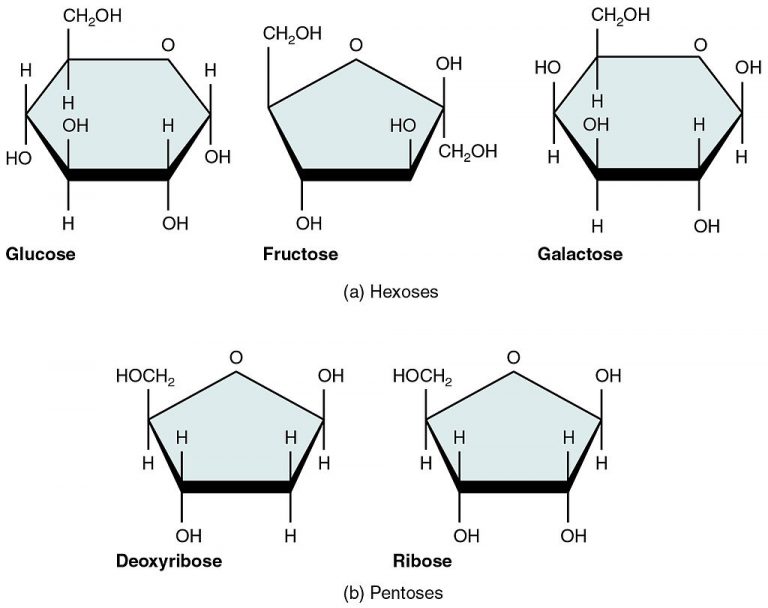
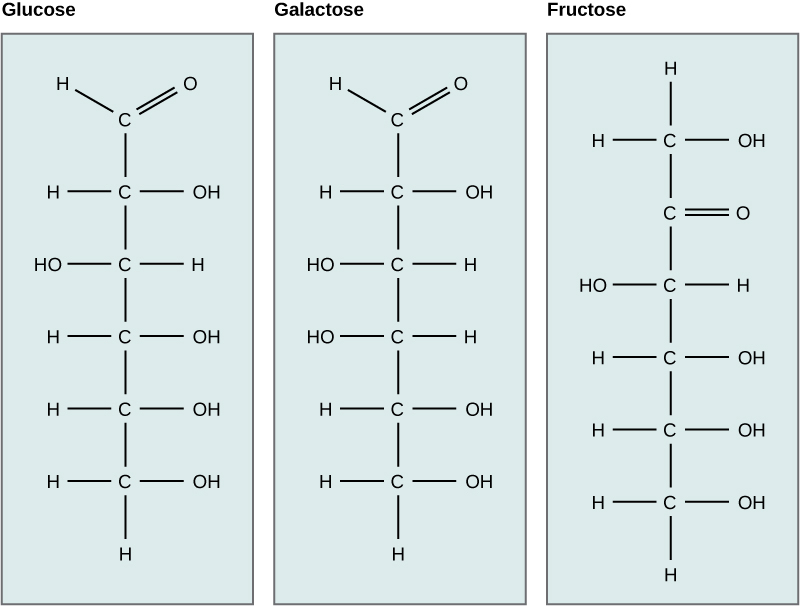
The chemical formula for glucose is C6H12O6. In most living species, glucose is an important source of energy. During cellular respiration, energy is released from glucose, which is used to help make adenosine triphosphate (ATP). Plants synthesize glucose using carbon dioxide and water through photosynthesis, and the glucose, in turn, is used for the plant’s energy requirements. The excess synthesized glucose is often stored as starch that is broken down by other organisms that feed on plants. Galactose (part of lactose or milk sugar) and fructose (found in fruit) are other common monosaccharides. Although glucose, galactose, and fructose all have the same chemical formula (C6H12O6), they differ structurally and chemically (known as isomers) because of differing arrangements of atoms in the carbon chain.
Disaccharides
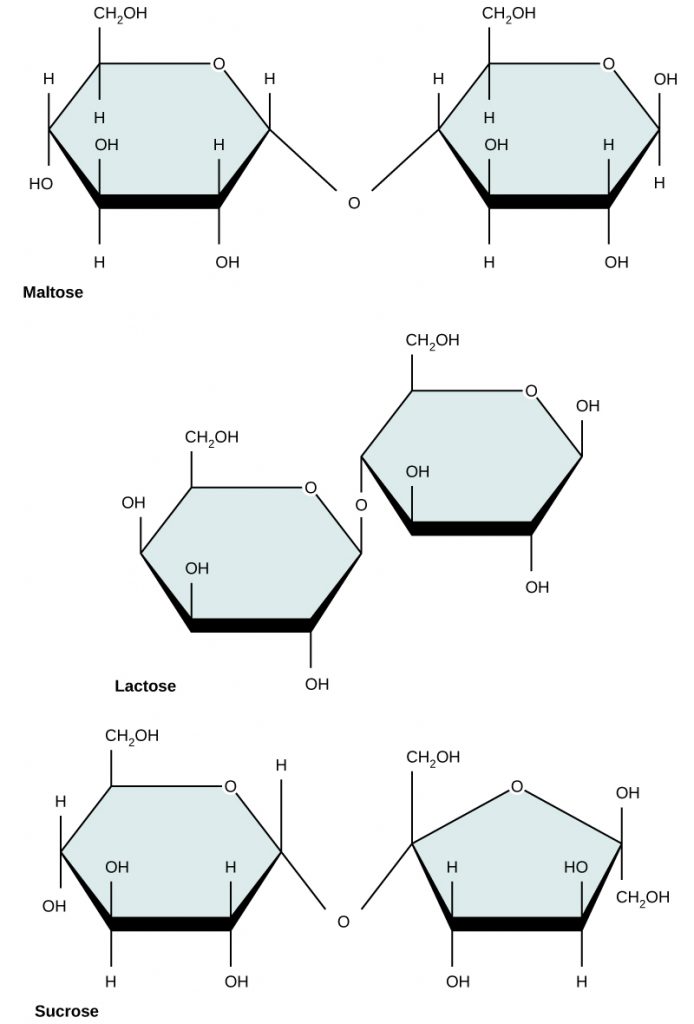
Disaccharides (di- = “two”) form when two monosaccharides undergo a dehydration reaction. During this process, the hydroxyl group (–OH) of one monosaccharide combines with a hydrogen atom of another monosaccharide, releasing a molecule of water (H2O) and forming a covalent bond between atoms in the two sugar molecules.
Lactose is a disaccharide consisting of the monomers glucose and galactose. It is found naturally in milk. Maltose, or malt sugar, is a disaccharide formed from a dehydration reaction between two glucose molecules. The most common disaccharide is sucrose, or table sugar, which is composed of the monomers glucose and fructose.
Polysaccharides
Some carbohydrates consist of hundreds — or even thousands! — of monosaccharides bonded together in long chains. These carbohydrates are called polysaccharides (“many saccharides”). Polysaccharides are also referred to as complex carbohydrates. Complex carbohydrates that are found in living things include starch, glycogen, cellulose, and chitin. Each type of complex carbohydrate has different functions in living organisms, but they generally either store energy or make up certain structures in living things.

Starch
Starch is a complex carbohydrate that is made by plants to store energy. For example, the potatoes pictured in Figure 3.2.4 are packed full of starches that consist mainly of repeating units of glucose and other simple sugars. The leaves of potato plants make sugars by photosynthesis, which are carried to underground tubers, where they are stored as starch. When we eat starchy foods such as potatoes, the starches are broken down by our digestive system into sugars, which provide our cells with energy. Starches are easily and quickly digested with the help of digestive enzymes such as amylase found in the saliva. If you chew a starchy saltine cracker for several minutes, you may start to taste the sugars released as the starch is digested.
Glycogen
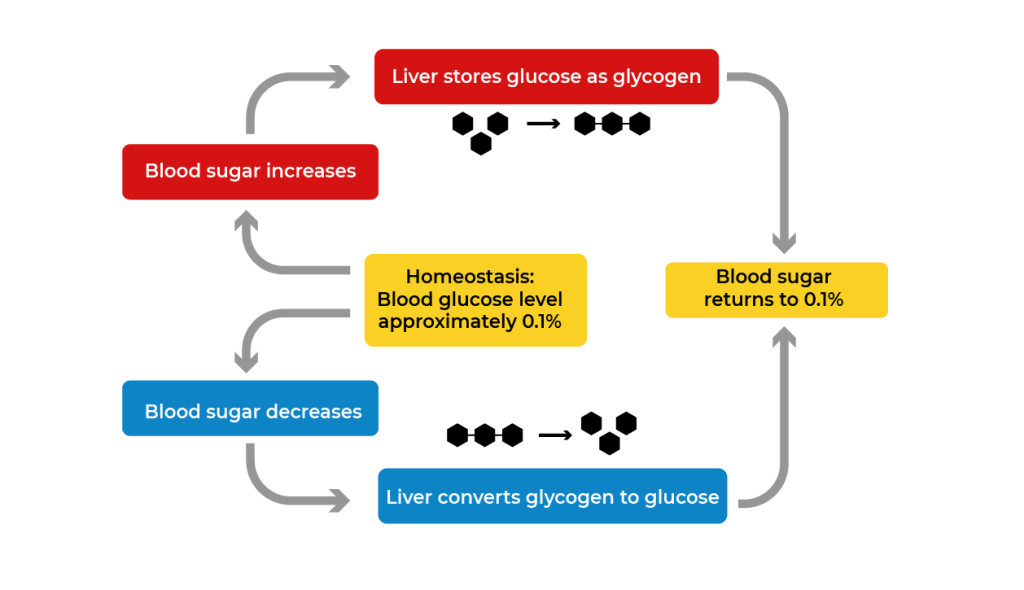
Animals do not store energy as starch. Instead, animals store extra energy as the complex carbohydrate glycogen. Glycogen is a polysaccharide of glucose. In humans, glycogen is made and stored primarily in the cells of the liver and muscles. When energy is needed, the glycogen is broken down to glucose for cell use. Muscle glycogen is converted to glucose for use by muscle cells, and liver glycogen is converted to glucose for use throughout the rest of the body. Glycogen forms an energy reserve that can be quickly mobilized to meet a sudden need for glucose, but one that is less compact than the energy reserves of lipids, which are the primary form of energy storage in animals.
Glycogen plays a critical part in the homeostasis of glucose levels in the blood. When blood glucose levels rise too high, excess glucose can be stored in the liver by converting it to glycogen. When glucose levels in the blood fall too low, glycogen in the liver can be broken down into glucose and released into the blood.
Cellulose

Cellulose is a polysaccharide with a linear chain of several hundred to many thousands of linked glucose units. Cellulose is an important structural component of plants and many algae cell walls. Human uses of cellulose include the production of cardboard and paper, which consist primarily of cellulose from wood and cotton. The cotton fibres pictured are about 90 percent cellulose.
Certain animals, including termites and ruminants such as cows, can digest cellulose with the help of microorganisms that live in their gut. Humans cannot digest cellulose, but it nonetheless plays an important role in our diet. It acts as a water-attracting bulking agent for feces in the digestive tract and is often referred to as “dietary fibre.” In simpler terms, it helps you poop.

Chitin
Chitin is a long-chain polymer of a derivative of glucose. It is found in many living things. For example, it is a component of the cell walls of fungi, the exoskeletons of arthropods, such as crustaceans and insects, and the beaks and internal shells of animals, such as squids and octopuses. The structure of chitin is similar to that of cellulose.
The Right Molecule for the Job
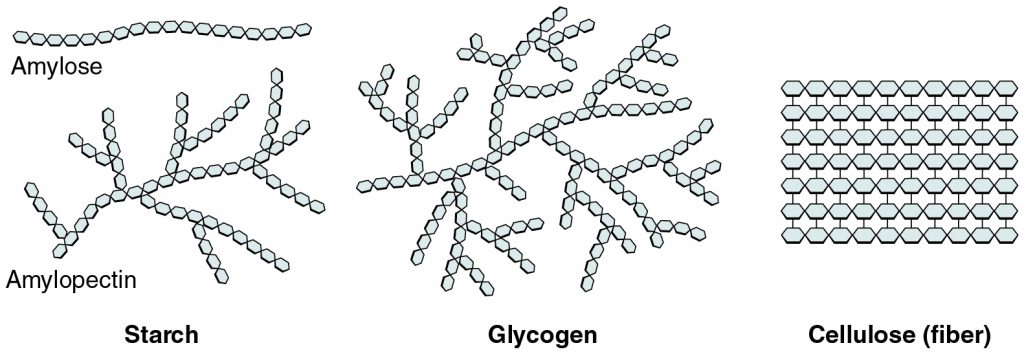
Starch, glycogen, cellulose, and chitin are all made from the monomer glucose. So, how are they all so different? Their difference in structure and function is related to how they are linked together. Starch is linked in long chains with a small amount of branching; glycogen is linked in many branches, and chitin and cellulose form long single chains that pack together tightly. These variations of linking the same monomer, glucose, create a different way the molecule can be used. As shown in Figure 3.2.8, starch and glycogen have many exposed “ends” of their chains. These are areas where a glucose molecule can easily be removed for use as energy, whereas cellulose does not. For this reason, glycogen and starch are well-suited for energy storage in organisms, while cellulose is not. Conversely, cellulose packs many monomers together in a very strong mesh — this is why it is a great option for building strong cell walls.
Exercise 3.2.1
Text Description
- _____ is a major component in the cell walls of fungi and the exoskeletons of insects.
- _____ is the storage form of glucose used by plants.
- _____ is the storage form of glucose used by animals.
- _____ is a major component in the cell walls of plants.
- Starch
- Chitin
- Cellulose
- Glycogen
Answers:
- Chitin is a major component in the cell walls of fungi and the exoskeletons of insects.
- Starch is the storage form of glucose used by plants.
- Glycogen is the storage form of glucose used by animals.
- Cellulose is a major component in the cell walls of plants.
“2.3 Biological Molecules” from Biology and the Citizen by Colleen Jones is licensed under a Creative Commons Attribution 4.0 International License, except where otherwise noted.
“3.4 Carbohydrates” from Human Biology by Christine Miller is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, except where otherwise noted.

