10.1 Genetic Engineering
Biotechnology uses artificial methods to modify the genetic material of living organisms or cells to produce novel compounds or perform new functions. Its roots can be traced back to ancient practices like fermentation for brewing and bread-making. It has also been used to improve livestock and crops since the beginning of agriculture through selective breeding. Modern biotechnology began to take shape in the 20th century with the discovery of DNA’s structure and the development of the basic tools used to manipulate DNA. This era saw the emergence of many different branches of biotechnology, including genetic engineering, stem cell research, and reproductive cloning.
Genetic engineering involves directly altering an organism’s DNA at the molecular level to achieve desired traits or outcomes. It plays a crucial role in medicine, agriculture, environmental science, and industry, offering solutions to the world’s most pressing challenges. By harnessing the power of living organisms, genetic engineering has the potential to improve health outcomes, enhance food security, and promote sustainable practices. The rate of discovery and development of new applications is expected to accelerate, bringing huge benefits to humankind but perhaps also significant risks. Many of these developments are expected to raise significant ethical and social questions that human societies have not yet had to consider.
Genetic engineering encompasses various techniques to manipulate an organism’s genetic material, including recombinant DNA technology and gene editing.
Isolation of Nucleic Acids
In order to manipulate the DNA, it first has to be removed, or extracted, from cells. This usually involves breaking the cell open and using enzymes to eliminate other large molecules. A detergent solution helps break the cells apart. Enzymes are used to stop proteins and RNA from interfering. Finally, alcohol is added to make the DNA come out of the solution, forming a jelly-like substance.
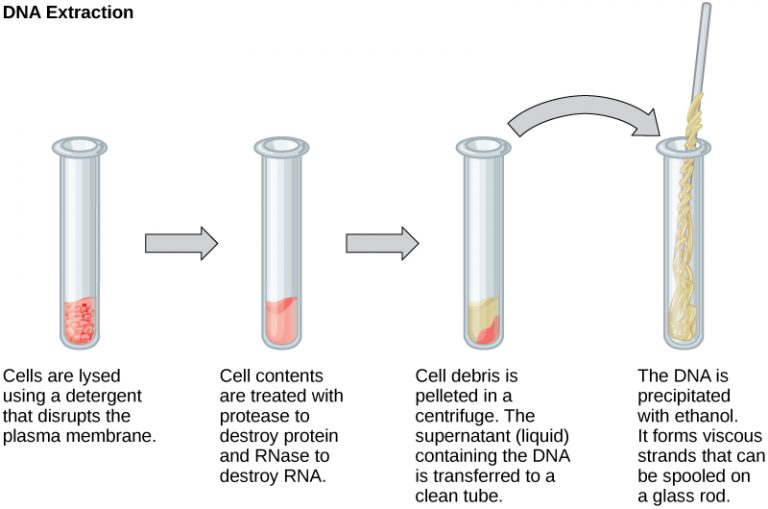
Figure 10.1.1 Image Description
This image illustrates the DNA extraction process in four key steps, using labelled diagrams of test tubes:
Cell Lysis: Cells are broken open using a detergent that disrupts the plasma membrane, releasing cellular contents into the solution.
Digestion of Proteins and RNA: The lysate is treated with protease to degrade proteins and RNase to break down RNA, leaving DNA intact.
Separation by Centrifugation: The mixture is centrifuged to pellet (collect) the debris at the bottom. The supernatant, which contains the DNA, is transferred to a new clean tube.
DNA Precipitation: Ethanol is added to the supernatant to precipitate the DNA. The DNA becomes visible as viscous strands that can be spooled on a glass rod.
Recombinant DNA technology
Recombinant DNA technology combines DNA from different sources to create new genetic combinations. A vector, a delivery vehicle for DNA, is used to help transport a specific DNA fragment into a cell for copying or expression. Bacterial cells are often used in this process, and the bacterial plasmid acts as the vector. A plasmid is a small circular DNA molecule found in bacteria that replicates independently of the larger bacterial chromosome. As bacteria divide, they copy their DNA, including the plasmid, which means the inserted DNA fragment is also copied. This process of making multiple identical copies of a specific gene is called gene cloning (or molecular cloning).
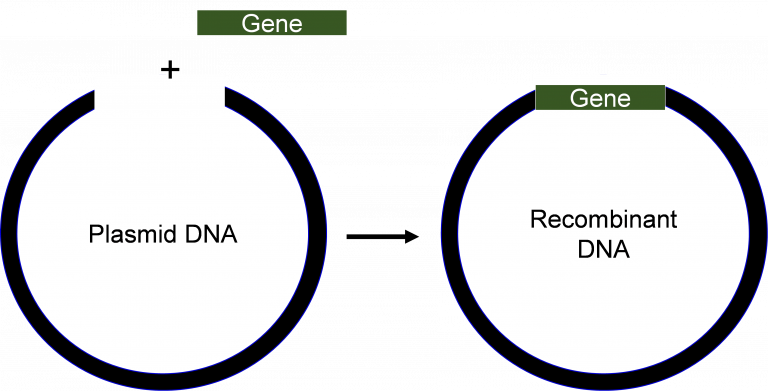
Gene cloning begins with the use of restriction enzymes, which act as molecular scissors to cut DNA at specific sequences. These enzymes recognize specific DNA sequences and cut them predictably, creating “sticky ends” that can bond with complementary overhangs on DNA cut with the same enzyme.
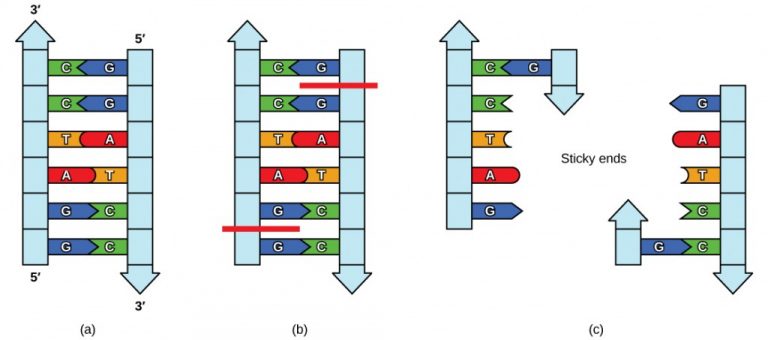
The sticky ends allow the isolated gene to be inserted into the plasmid. DNA ligase joins them together, forming recombinant plasmids. The recombinant plasmid is added to a host cell and typically then cultured to produce large quantities of the desired gene.
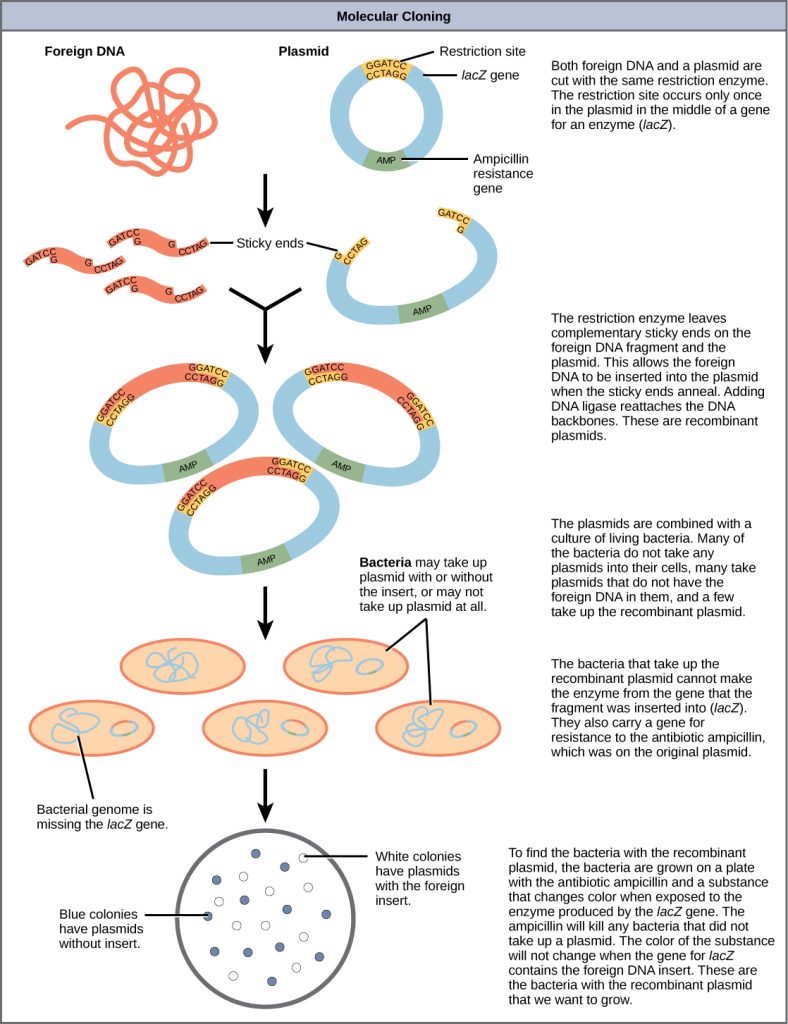
Figure 10.1.4 Image Description
This image illustrates the molecular cloning process using plasmids and bacterial transformation. It breaks the procedure into multiple stages, from cutting DNA to identifying transformed bacteria:
1. DNA Cutting and Preparation:
- Foreign DNA and a plasmid are cut using the same restriction enzyme at a specific restriction site (within the lacZ gene).
- The plasmid also contains an ampicillin resistance gene (Amp).
- Cutting both DNA sources creates complementary sticky ends.
2. DNA Insertion
- The foreign DNA is inserted into the plasmid via sticky end pairing.
- DNA ligase is used to seal the DNA backbones.
- The resulting recombinant plasmids now contain the foreign DNA.
3. Transformation
- These plasmids are introduced to a bacterial culture.
- Bacteria may:
- Take up plasmids with foreign DNA (recombinant plasmids),
- Take up plasmids without the insert,
- Or not take up any plasmid.
4. Bacterial Selection
- Bacteria are plated on media containing ampicillin and a substance that detects activity of the lacZ gene.
- Only bacteria with plasmids (containing ampicillin resistance) survive.
5. Screening Colonies
- Blue colonies: Bacteria with plasmids without the foreign DNA insert (lacZ is intact and active).
- White colonies: Bacteria with recombinant plasmids (lacZ is disrupted by foreign DNA).
- These white colonies are the ones that successfully incorporated the foreign gene of interest and are typically selected for further study or application.
Applications of Recombinant DNA Technology
Recombinant DNA technology has many different applications. Some examples include:
Insulin Production
Human genes can be inserted into bacteria so they are able to make human proteins. Proteins the bacteria make are injected into people who cannot produce them because of mutations.
Insulin was the first human protein to be produced in this way. Insulin helps cells take up glucose from the blood. People with type 1 diabetes have a mutation in the gene that usually codes for insulin.
Without insulin, their blood glucose rises to harmfully high levels. At present, the only treatment for type 1 diabetes is the injection of insulin from outside sources. Until recently, there was no known way to make human insulin outside the human body. The problem was solved using recombinant DNA technology. The human insulin gene was cloned and used to transform bacterial cells, which could then produce large quantities of human insulin. Other human proteins, such as Human Growth Hormone and cytokines, have been produced this way.
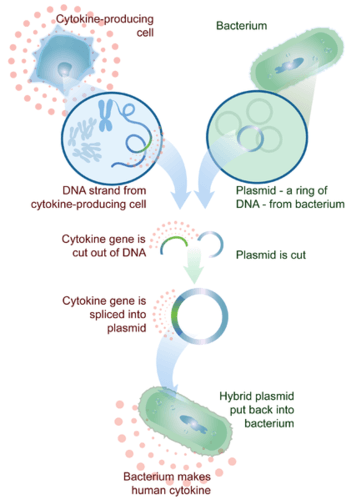
Figure 10.1.5 Image Description
This image illustrates the recombinant DNA process used to produce human cytokines using bacteria:
Step-by-Step Description:
- Cytokine-Producing Cell:
A human or animal cell that naturally produces cytokines is selected. Its DNA is extracted. - Gene Isolation:
The specific cytokine gene is cut out of the DNA using restriction enzymes. - Plasmid from Bacterium:
A plasmid, which is a small circular piece of DNA from a bacterium, is also cut open. - Recombination:
The cytokine gene is inserted (spliced) into the plasmid, forming a hybrid (recombinant) plasmid. - Transformation:
The recombinant plasmid is inserted back into a bacterium. - Protein Production:
The bacterium now uses the inserted gene to produce human cytokine, which can be harvested for medical use.
This image shows how genetic engineering allows for the mass production of human proteins (like cytokines) in microorganisms—a process commonly used in biotechnology and pharmaceutical industries.
Transgenic Animals
Although several recombinant proteins used in medicine are successfully produced in bacteria, some proteins need a eukaryotic animal host for proper processing. For this reason, genes have been cloned and expressed in animals such as sheep, goats, chickens, and mice. Animals that have been modified to express recombinant DNA are called transgenic animals.
Several human proteins are expressed in the milk of transgenic sheep and goats. In one commercial example, the FDA has approved a blood anticoagulant protein that is produced in the milk of transgenic goats for use in humans. Mice have been used extensively to express and study the effects of recombinant genes and mutations.

The first transgenic animal was approved for food in Canada in 2016. The AquAdvantage salmon incorporates a growth hormone gene from Chinook salmon into the genome of Atlantic salmon. This results in a salmon which grows faster and reaches market size quicker.
Transgenic Crops
Transgenic crops, also known as genetically modified organisms (GMOs), are modified with new genes that code for traits helpful to humans.
Transgenic crops have been created with a variety of different traits. They can yield more food, taste better, survive drought, tolerate salty soil, and resist insect pests and diseases, among other things. There are hundreds of GM crops approved in Canada. One example is Bt corn, which is a type of GMO that has been engineered to produce protein from the bacterium Bacillus thuringiensis (Bt). This protein is toxic to certain insect pests, particularly caterpillars like the European corn borer. Bt corn helps farmers reduce the need for chemical insecticides. Scientists have even created a transgenic purple tomato that contains high levels of cancer-fighting compounds called antioxidants.

Production of Vaccines
A vaccine is a substance that stimulates the immune system to recognize and fight specific pathogens, providing protection against disease.
Traditional vaccines use weakened or inactive microorganisms or viruses to trigger an immune response. Modern vaccines use specific genes from pathogens, which are cloned and mass-produced in bacteria to create large quantities of specific proteins that stimulate the immune system. These proteins are then used as the vaccine.
Traditionally, flu vaccines (aka flu shots) were made by growing the virus in chicken eggs, which was a time-consuming and labour-intensive process. With recombinant DNA technology, flu vaccines can be produced faster and quickly adapted to respond to emerging flu strains.
Gene Therapy
Gene therapy is a recombinant DNA technique that aims to cure certain genetic diseases by introducing a healthy gene into the genome. This process involves replacing a faulty or missing protein caused by a genetic mutation. The healthy gene is typically delivered into diseased cells using a vector, such as a virus (e.g., adenovirus), which can infect the host cell and integrate the foreign DNA into the genome.
While gene therapy has primarily been experimental, several treatments have recently been approved in Canada. For example, Hemgenix is a hemophilia treatment that delivers a functional copy of the gene responsible for producing Factor IX, a protein essential for blood clotting. As technology advances and challenges are addressed, gene therapy holds promise for curing more genetic diseases in the future.
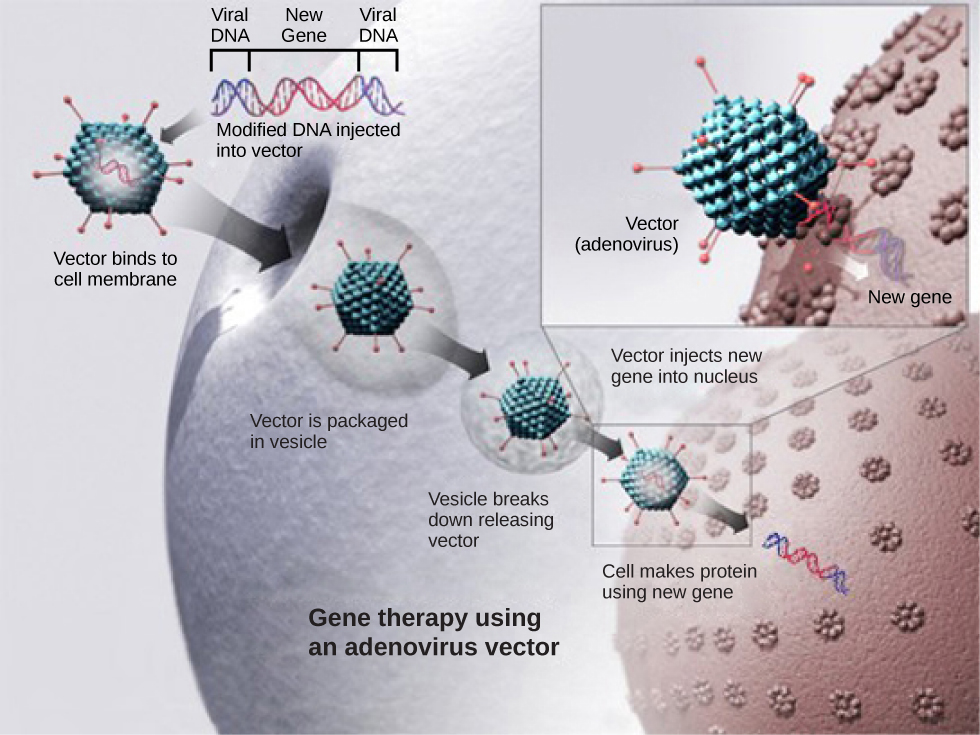
Figure 10.1.8 Image Description
This image illustrates the process of gene therapy using an adenovirus vector, which is a common method for delivering therapeutic genes into human cells.
Step-by-Step Description:
- Modified DNA Injected into Vector
A therapeutic gene (new gene) is inserted into the DNA of an adenovirus. The modified viral DNA now carries both viral DNA and the new gene. - Vector Binds to Cell Membrane
The engineered adenovirus (vector) comes into contact with a human cell and binds to its membrane. - Vector is Packaged in a Vesicle
The virus is taken into the cell by endocytosis, forming a vesicle around it. - Vesicle Breaks Down
Inside the cell, the vesicle breaks open, releasing the vector into the cytoplasm. - Vector Injects New Gene into Nucleus
The virus travels to the nucleus and delivers the new gene into the cell’s genome or as an episome (non-integrated DNA). - Protein Production
The cell uses the new gene to produce the corresponding protein, which may help treat a genetic disorder or disease.
Purpose:
Gene therapy using adenovirus vectors is designed to correct or replace faulty genes by delivering functional copies directly into a patient’s cells.
Gene Editing
Gene editing is another technique used in genetic engineering. Gene editing allows for precise changes to a gene directly inside living organisms. Gene editing can be used to add, delete or even substitute bases within the DNA. In contrast, we saw that recombinant DNA technology can only be used to add a gene to an organism.
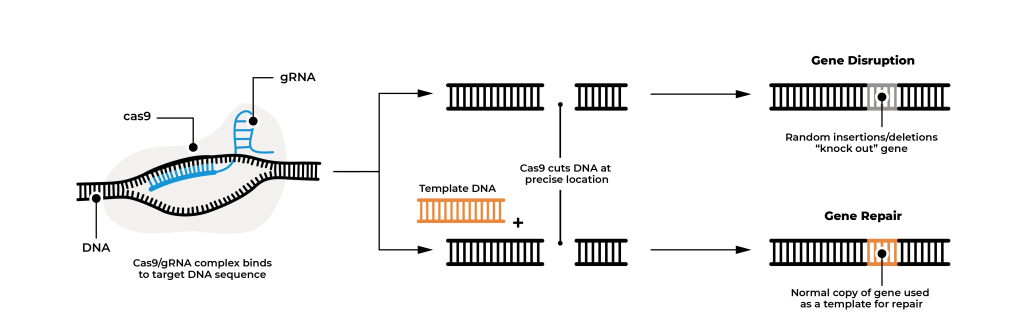
The most revolutionary tool in gene editing is CRISPR-Cas9. Discovered in 2012 by Jennifer Doudna and Emmanuelle Charpentier, CRISPR-Cas9 has transformed the field by providing a simple, efficient, and accurate method for targeting specific DNA sequences. CRISPR-Cas9 is a natural system found in bacteria which serves as a defence mechanism against viruses called bacteriophages. When a bacteriophage infects a bacterium, the CRISPR system captures snippets of the virus’s DNA and integrates them into the bacterial genome. These snippets are then used to recognize and cut out the DNA of future infections by the same virus, thus providing immunity.
CRISPR-Cas9 involves two main components: the Cas9 enzyme, which acts as molecular scissors to cut DNA, and a guide RNA (gRNA) that is complementary to the specific DNA sequence to be edited. After Cas9 cuts both strands of the target DNA, the cell’s DNA repair enzymes randomly insert or delete nucleotides as they reconnect the DNA. This process can disrupt the gene’s function, effectively “knocking out” the gene.
Scientists can also introduce a normal gene into the cell with the Cas9/gRNA complex to be used as a template to repair the cut DNA. Using a DNA template allows for precise corrections or insertions at the cut site, enabling the repair of genetic mutations or the addition of new genetic material.
Applications of Gene Editing
CRISPR-Cas9 technology has a wide range of applications across various fields. Many of these applications are still in the research and development stage, but some have been approved.
Medical Applications
CRISPR-Cas9 technology holds immense potential in various medical applications. In gene therapy, CRISPR-Cas9 can be used to cure genetic disorders by precisely editing the mutations in genes. The first CRISPR-Cas9 gene-edited therapy was approved in Canada in 2024 for sickle cell disease and transfusion-dependent beta-thalassemia. It involves editing hematopoietic stem cells (stem cells found in the bone marrow that can develop into all of the different types of blood cells) to correct genetic mutations.
In cancer treatment, CRISPR-Cas9 can target and modify genes involved in cancer progression, potentially leading to more effective and personalized therapies. Researchers are exploring ways to use this technology to disrupt cancer cell growth and enhance the body’s ability to fight cancer.
CRISPR-Cas9 can also be used to develop treatments for viral infections like HIV and hepatitis B. This approach aims to target and remove viral DNA from infected cells, potentially offering a cure for these chronic infections.
Agricultural Applications

CRISPR-Cas9 technology is used extensively in agriculture. In crops, CRISPR can modify specific genes to enhance resistance to diseases, pests, and environmental stresses. For example, researchers have developed CRISPR-edited wheat varieties that are more resistant to fungal infections and drought conditions. CRISPR can also be used to improve the nutritional content of crops, such as increasing the levels of essential vitamins and minerals. The Canadian Food Inspection Agency (CFIA) and Health Canada have established guidelines that treat gene-edited crops similarly to traditionally bred varieties. This means that gene-edited crops do not require the same stringent pre-market safety evaluations as genetically modified organisms (GMOs) with foreign DNA. In livestock, CRISPR is being explored to enhance traits like disease resistance, growth rates, and overall productivity.
Environmental Applications
Researchers are exploring many possible environmental applications of CRISPR gene editing. In bioremediation, CRISPR is used to modify the genes of microorganisms that can break down pollutants and toxins in the environment more efficiently. Research is underway to try to modify the genes of certain bacteria to enhance their ability to degrade plastics and help solve the global plastic waste problem.
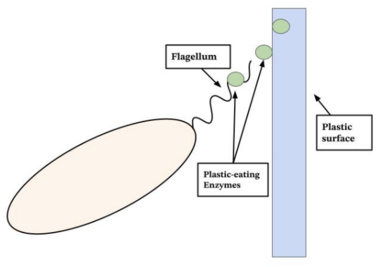
In conservation, CRISPR technology is being explored as a way to help protect endangered species by enhancing their genetic diversity. This can be achieved by introducing specific genetic variants from global populations or even from museum specimens to help improve their resilience to diseases and environmental changes.
One day, CRISPR may be used to tackle invasive species by editing genes responsible for reproduction or survival, helping restore balance in ecosystems.
Potential Application – CRISPR-Cas 9 Gene Drives
A promising application of CRISPR-Cas9 technology is the development of gene drives. A gene drive is a genetic system designed to spread a specific trait through a population much faster than normal inheritance would allow. Under normal Mendelian inheritance, each parent has a 50% chance of passing a gene to their offspring. A gene drive changes this by biasing inheritance, ensuring that the desired gene is passed on to nearly all offspring.
Gene drives work by inserting both the desired gene and the CRISPR-Cas9 machinery into an organism’s DNA. When the organism reproduces, its offspring inherit one copy of the gene drive. In the offspring, CRISPR-Cas9 cuts the matching site on the other chromosome, and the cell repairs the cut by copying the gene drive into that location. This results in the offspring having two copies of the gene drive, allowing the trait to spread rapidly through the population.
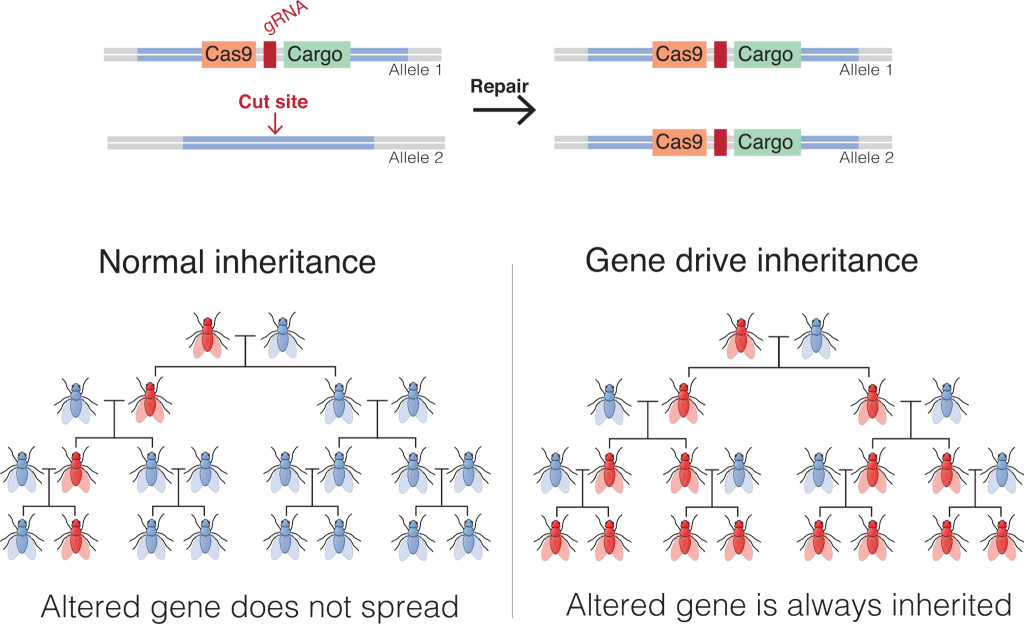
Figure 10.1.13 Image Description
This image explains the concept of gene drive inheritance using the CRISPR-Cas9 system, and compares it to normal inheritance.
Top Section: Mechanism of Gene Drive
- Cas9 and gRNA (guide RNA) are inserted into Allele 1 alongside a “Cargo” gene (the desired genetic modification).
- Cas9 cuts the corresponding Allele 2 at the target site.
- During repair, the cell copies the entire Cas9-Cargo sequence from Allele 1 into Allele 2.
- Result: Both alleles now carry the altered gene—this is the gene drive.
Bottom Section: Inheritance Patterns
- Normal Inheritance (Left)
- Shows how a genetically altered gene may not be passed on to all offspring.
- Over generations, the altered gene may disappear.
- Conclusion: “Altered gene does not spread.”
- Gene Drive Inheritance (Right)
- Due to the gene drive, every offspring inherits the altered gene.
- The trait rapidly spreads through the population.
- Conclusion: “Altered gene is always inherited.”
Gene drives are currently being studied in controlled environments to evaluate their safety and effectiveness. Potential applications include:
- Reduce the spread of vector-borne diseases like malaria, dengue, and Lyme Disease. For example, in the case of malaria, a gene drive can be used to either make the mosquitoes (Anopheles) sterile or make them resistant to the parasite (Plasmodium).
- Manage invasive species by reducing their ability to reproduce.
- Eliminate pesticide resistance in agricultural pests.
Despite their potential, gene drives also raise significant concerns, including unintended ecological consequences, the difficulty of reversing genetic changes once they have spread once released in the wild, and ethical questions about altering entire populations.
“10.1 Cloning and Genetic Engineering” from Biology and the Citizen by Colleen Jones is licensed under a Creative Commons Attribution 4.0 International License, except where otherwise noted.
“Recombinant DNA Technology” from Genetics, Agriculture, and Biotechnology by Patricia Hain is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License, except where otherwise noted.
“5.16 Genetic Engineering” from Human Biology by Christine Miller is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, except where otherwise noted.
“10.2 Biotechnology in Medicine and Agriculture” from Biology and the Citizen by Colleen Jones is licensed under a Creative Commons Attribution 4.0 International License, except where otherwise noted.

