34 5.2 Chemical Weathering
Chemical weathering results from chemical changes to minerals that become unstable when they are exposed to surface conditions. The kinds of changes that take place are highly specific to the mineral and the environmental conditions. Some minerals, like quartz, are virtually unaffected by chemical weathering, while others, like feldspar, are easily altered. In general, the degree of chemical weathering is greatest in warm and wet climates, and least in cold and dry climates. The important characteristics of surface conditions that lead to chemical weathering are the presence of water (in the air and on the ground surface), the abundance of oxygen, and the presence of carbon dioxide, which produces weak carbonic acid when combined with water. That process, which is fundamental to most chemical weathering, can be shown as follows:
H2O + CO2 —->H2CO3 then H2CO3 —-> H+ + HCO3–,
water + carbon dioxide —-> carbonic acid then carbonic acid —-> hydrgen ion + carbonate ion
Here we have water (e.g., as rain) plus carbon dioxide in the atmosphere, combining to create carbonic acid. Then carbonic acid dissociates (comes apart) to form hydrogen and carbonate ions. The amount of CO2 in the air is enough to make only very weak carbonic acid, but there is typically much more CO2 in the soil, so water that percolates through the soil can become significantly more acidic.
There are two main types of chemical weathering. On the one hand, some minerals become altered to other minerals. For example, feldspar is altered — by hydrolysis — to clay minerals. On the other hand, some minerals dissolve completely, and their components go into solution. For example, calcite (CaCO3) is soluble in acidic solutions.
The hydrolysis of feldspar can be written like this:
CaAl2Si2O8 + H2CO3 + ½O2 —-> Al2Si2O5(OH)4 + Ca2+ + CO32-
plagioclase + carbonic acid —-> kaolinite + dissolved calcium + carbonate ions
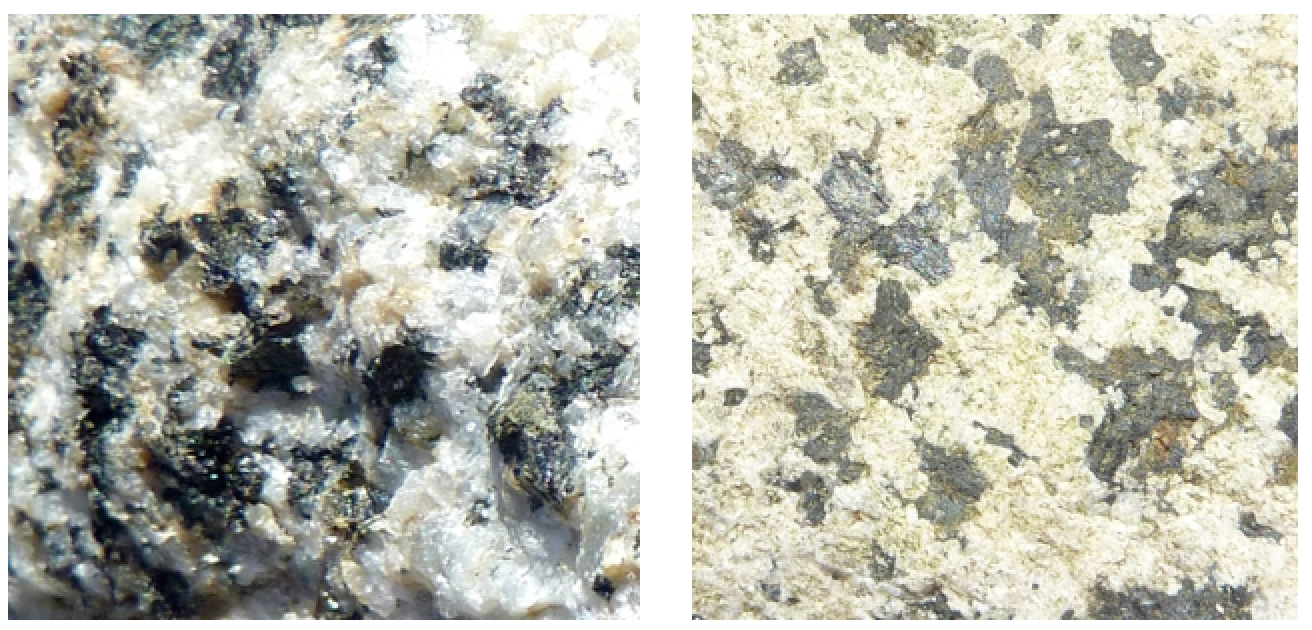
This reaction shows calcium plagioclase feldspar, but similar reactions could also be written for sodium or potassium feldspars. In this case, we end up with the mineral kaolinite, along with calcium and carbonate ions in solution. Those ions can eventually combine (probably in the ocean) to form the mineral calcite. The hydrolysis of feldspar to clay is illustrated in Figure 5.9, which shows two images of the same granitic rock, a recently broken fresh surface on the left and a clay-altered weathered surface on the right. Other silicate minerals can also go through hydrolysis, although the end results will be a little different. For example, pyroxene can be converted to the clay minerals chlorite or smectite, and olivine can be converted to the clay mineral serpentine.
Oxidation is another very important chemical weathering process. The oxidation of the iron in a ferromagnesian silicate starts with the dissolution of the iron. For olivine, the process looks like this, where olivine in the presence of carbonic acid is converted to dissolved iron, carbonate, and silicic acid:
Fe2SiO4+ 4H2CO3 —> 2Fe2+ + 4HCO3– + H4SiO4
olivine + (carbonic acid) —> dissolved iron + dissolved carbonate + dissolved silicic acid
In the presence of oxygen, the dissolved iron is then quickly converted to hematite:
2Fe2+ + 4HCO3– + ½ O2 + 2H2O —->Fe2O3 + 4H2CO3
dissolved iron + bicarbonate + oxygen + water—->hematite + carbonic acid
The equation shown here is for olivine, but it could apply to almost any other ferromagnesian silicate, including pyroxene, amphibole, or biotite. Iron in the sulphide minerals (e.g., pyrite) can also be oxidized in this way. And the mineral hematite is not the only possible end result, as there is a wide range of iron oxide minerals that can form in this way. The results of this process are illustrated in Figure 5.10, which shows a granitic rock in which some of the biotite and amphibole have been altered to form the iron oxide mineral limonite.

A special type of oxidation takes place in areas where the rocks have elevated levels of sulphide minerals, especially pyrite (FeS2). Pyrite reacts with water and oxygen to form sulphuric acid, as follows:
2FeS2 + 7O2 +2H2O —–> 2Fe2+ H2SO4+ 2H+
pyrite + oxygen + water —–> iron ions + sulphuric acid + hydrogen ions
The runoff from areas where this process is taking place is known as acid rock drainage (ARD), and even a rock with 1% or 2% pyrite can produce significant ARD. Some of the worst examples of ARD are at metal mine sites, especially where pyrite-bearing rock and waste material have been mined from deep underground and then piled up and left exposed to water and oxygen. One example of that is the Mt. Washington Mine near Courtenay on Vancouver Island (Figure 5.11), but there are many similar sites across Canada and around the world.

At many ARD sites, the pH of the runoff water is less than 4 (very acidic). Under these conditions, metals such as copper, zinc, and lead are quite soluble, which can lead to toxicity for aquatic and other organisms. For many years, the river downstream from the Mt. Washington Mine had so much dissolved copper in it that it was toxic to salmon. Remediation work has since been carried out at the mine and the situation has improved.
The hydrolysis of feldspar and other silicate minerals and the oxidation of iron in ferromagnesian silicates all serve to create rocks that are softer and weaker than they were to begin with, and thus more susceptible to mechanical weathering.
The weathering reactions that we’ve discussed so far involved the transformation of one mineral to another mineral (e.g., feldspar to clay), and the release of some ions in solution (e.g., Ca2+). Some weathering processes involve the complete dissolution of a mineral. Calcite, for example, will dissolve in weak acid, to produce calcium and bicarbonate ions. The equation is as follows:
CaCO3 + H+ + HCO3– —–> Ca2+ + 2HCO3–
calcite + hydrogen ions + bicarbonate —–> calcium ions + bicarbonate
Calcite is the major component of limestone (typically more than 95%), and under surface conditions, limestone will dissolve to varying degrees (depending on which minerals it contains, other than calcite), as shown in Figure 5.12. Limestone also dissolves at relatively shallow depths underground, forming limestone caves. This is discussed in more detail in Chapter 14, where we look at groundwater.

Exercises
Exercise 5.2 Chemical Weathering
The main processes of chemical weathering are hydrolysis, oxidation, and dissolution. Complete the following table by indicating which process is primarily responsible for each of the described chemical weathering changes:
| Chemical Change | Process? |
| Pyrite to hematite | |
| Calcite to calcium and bicarbonate ions | |
| Feldspar to clay | |
| Olivine to serpentine | |
| Pyroxene to iron oxide |

